Cardiovascular Inflammaging: Understanding How Chronic Inflammation Ages Your Heart
Chronic inflammation (inflammaging) is an independent heart disease risk. Discover how senescent cells and immunosenescence age your heart and the best anti-inflammatory strategies.
HEART
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/3/202612 min read
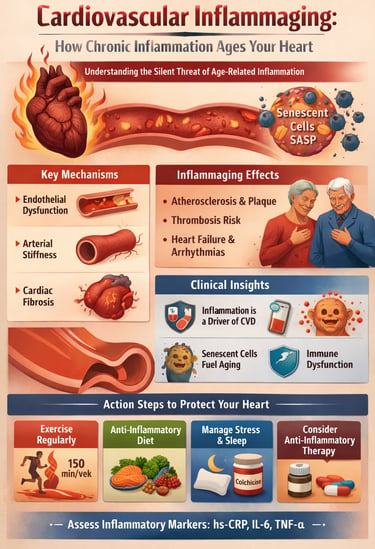
Your heart does not fail suddenly with age—it is slowly reshaped, year after year, by a biological process most people never feel and few clinicians routinely measure. Long before the first plaque ruptures or blood pressure rises, a silent form of chronic inflammation begins to erode cardiovascular structure and function. This process, now known as cardiovascular inflammaging, represents one of the most important paradigm shifts in modern cardiology (Spray et al., 2025).
Unlike acute inflammation, which protects the body from injury and infection, inflammaging is a state of persistent, low-grade immune activation that accompanies aging itself. Circulating inflammatory mediators such as interleukin-6, tumor necrosis factor-α, and C-reactive protein gradually rise over decades, quietly impairing endothelial function, stiffening arteries, and altering cardiac tissue architecture (Bartoli-Leonard et al., 2025). Crucially, this inflammatory burden predicts cardiovascular events independently of traditional risk factors—including LDL cholesterol, blood pressure, and body mass index—helping explain why many individuals with “optimal” numbers still suffer myocardial infarction or stroke.
At the cellular level, aging immune systems undergo profound dysregulation. Immunosenescence reduces effective immune surveillance while paradoxically amplifying inflammatory signaling, creating a state of simultaneous immune deficiency and hyperinflammation (Ajoolabady et al., 2024). Senescent cells accumulate within vascular and cardiac tissue, secreting a toxic mix of cytokines and proteases—the senescence-associated secretory phenotype—that accelerates atherosclerosis, fibrosis, and thrombosis (Zeng et al., 2025).
Taken together, these discoveries redefine cardiovascular aging not as passive wear and tear, but as an active, immune-driven disease process. Understanding inflammaging is no longer optional—it is essential for explaining residual cardiovascular risk, guiding prevention strategies, and shaping the future of heart disease management in aging populations.
Clinical pearls
1 Inflammation is an Independent Cardiovascular Risk Driver: Chronic, low-grade systemic inflammation, often referred to as inflammaging, is not just a secondary marker but an independent, primary driver of cardiovascular disease (CVD), even in individuals with optimal traditional risk factors
Inflammation is the "Hidden Fire": Even if your cholesterol is normal, chronic inflammation can act like a slow-burning fire in your arteries. Testing for markers like CRP can show if you have a "hidden risk" that standard tests miss.
2 Senescent Cell Burden & SASP: The accumulation of non-dividing "zombie" cells drives vascular aging. These cells secrete the Senescence-Associated Secretory Phenotype (SASP), a cocktail of cytokines that directly induces endothelial dysfunction and cardiac fibrosis.
As we age, some cells stop working but don't leave. These "zombie cells" release toxins that stiffen your heart and blood vessels. New science is looking for ways to clear these out to keep your heart young.
3 Vascular Wall Autonomy: Arterial stiffening is an active biochemical process. Endothelial and smooth muscle cells are not passive victims; they actively amplify the inflammatory cycle, making the vascular wall itself a key therapeutic target.
Your blood vessels are alive and constantly reacting. They don't just wear out like old pipes; they actively participate in your health. Keeping them "calm" through good habits prevents them from becoming stiff and brittle.
4 Multi-Modal Lifestyle Intervention: Evidence-based lifestyle changes (Mediterranean diet, zone-2 and resistance exercise) remain the most potent "epigenetic" tools to downregulate systemic inflammatory signaling across multiple pathways.
Exercise and a plant-rich diet aren't just for weight loss; they act like a "clean-up crew" for your blood vessels, lowering the chemicals that cause inflammation and aging.
5 The Immunosenescence Paradox: Aging creates a dual-threat state of hyperinflammation and immunodeficiency. Management requires immune modulation to restore homeostatic balance rather than broad immunosuppression.
As we get older, our immune system gets confused—it’s too weak to fight off germs but too "cranky," causing constant internal irritation. We want to help it find its balance so it protects you instead of attacking your heart.
Understanding Inflammaging: The Cellular Root of Cardiovascular Decline
Inflammaging represents a fundamental shift in immune function that occurs with advancing age (Ajoolabady et al., 2024). Rather than mounting effective, targeted responses to threats, your aging immune system becomes primed for a state of chronic, systemic inflammation. Think of it as your body's security system developing a hair-trigger sensitivity—it fires constantly and indiscriminately, even in the absence of genuine threats.
This chronic inflammatory state is characterized by elevated circulating levels of pro-inflammatory cytokines, including TNF-α (tumor necrosis factor-alpha), IL-6 (interleukin-6), and CRP (C-reactive protein) (Bartoli-Leonard et al., 2025). These molecular messengers, typically beneficial in controlled amounts, become problematic when persistently elevated.
The Immunosenescence Connection
Immunosenescence describes the progressive deterioration of immune function with age. According to recent comprehensive research, key features include thymic involution (shrinkage of the thymus gland), reduced production of new T cells, impaired B cell function, altered innate immune responses, and accumulation of senescent cells (Ajoolabady et al., 2024).
The breakdown of immune tolerance and dysregulation of inflammatory responses create a perfect storm for cardiovascular disease. As your immune system ages, it loses its ability to distinguish friend from foe, attacking healthy tissue while simultaneously becoming vulnerable to infections (Spray et al., 2025). This paradox—simultaneous immunodeficiency and hyperinflammation—defines the immunosenescent state. For the cardiovascular system, the consequences are particularly severe.
The Cardiovascular Toll: How Inflammation Ages Your Heart
Mechanisms of Cardiovascular Inflammaging
A recent comprehensive analysis by Zeng et al. (2025) about cardiovascular inflammaging mechanisms reveals multiple pathways through which chronic inflammation damages the heart and vasculature :
1. Endothelial Dysfunction
The endothelium—your blood vessels' innermost lining—serves as a barrier and regulator of vascular function. Pro-inflammatory cytokines compromise endothelial integrity, reducing the production of nitric oxide (a critical vasodilator) and increasing oxidative stress. This triggers a cascade of vascular damage, promoting atherosclerosis and hypertension.
2. Arterial Stiffness and Vascular Remodeling
Chronic inflammation accelerates vascular aging through enhanced collagen deposition and arterial wall remodelling. Your arteries become less compliant, forcing your heart to work harder and contributing to systolic hypertension and heart failure. Research into vascular inflammaging mechanisms demonstrates that inflammatory mediators directly promote the structural changes that stiffen arteries
3. Cardiac Fibrosis and Diastolic Dysfunction
Inflammatory cytokines stimulate cardiac fibroblasts to produce excessive collagen, leading to cardiac fibrosis. This stiffens the heart muscle, impairing its ability to relax and fill with blood during diastole. Many older adults develop diastolic dysfunction—reduced relaxation capacity—years before systolic problems emerge.
4. Atherosclerosis Acceleration
Chronic inflammation is a fundamental driver of atherosclerotic plaque formation and progression. Pro-inflammatory macrophages infiltrate arterial walls, lipids accumulate, and plaques destabilise, increasing risk for myocardial infarction and stroke
5. Thrombotic Risk
Inflammaging promotes a prothrombotic state—increased tendency for blood clots. Inflammatory mediators activate platelets and endothelial cells to express tissue factor, while simultaneously impairing natural anticoagulant mechanisms (Spray et al., 2025).
6. Autonomic Dysfunction
Aging and inflammation impair the autonomic nervous system's ability to regulate heart rate and blood pressure, reducing heart rate variability and increasing susceptibility to arrhythmias (Ajoolabady et al., 2024).
The Role of Senescent Cells
One particularly fascinating mechanism involves senescent cells—aging cells that stop dividing but refuse to die. These cellular zombies accumulate with age and, crucially, secrete a toxic cocktail of pro-inflammatory factors called the senescence-associated secretory phenotype (SASP). This SASP directly fuels cardiovascular inflammaging, promoting endothelial dysfunction, vascular stiffness, and atherosclerosis. Recent findings suggest that targeting senescent cells through senolytic therapy (drugs that eliminate senescent cells) may offer promising cardiovascular benefits
The Hidden Burden: Understanding Inflammation's Cardiovascular Impact
Here's a troubling reality: Many people with normal cholesterol, optimal blood pressure, and ideal BMI still suffer heart attacks and strokes. Why? Because traditional cardiovascular risk factors don't capture the full picture of disease risk.
Research examining the hidden burden of inflammation and cardiovascular disease demonstrates that chronic inflammation independently predicts cardiovascular events, even after accounting for conventional risk factors (Bartoli-Leonard et al., 2025). This means that even if you've optimized your cholesterol, blood pressure, and weight, if your inflammatory markers remain elevated, your heart remains at risk.
High-sensitivity CRP (hs-CRP), IL-6, and TNF-α emerge as independent predictors of cardiovascular events. The role of the immune system in cardiovascular health and disease goes far beyond the occasional inflammatory flare—it's a constant, underlying factor shaping your heart's destiny
Age as the Silent Amplifier
Age itself isn't just a number; it's a biological reality that exponentially increases cardiovascular risk. The interplay between aging, inflammation, and cardiovascular disease creates a particularly precarious situation for older adults . A 75-year-old has fundamentally different immune and cardiovascular physiology than a 45-year-old, even with identical modifiable risk factors.
This age-related amplification of disease risk reflects the cumulative burden of inflammaging. The longer your cardiovascular system has been exposed to chronic inflammatory conditions, the greater the structural and functional damage
Latest Research Insights: What the Science Reveals
Insight 1: Inflammation is an Independent Disease Driver
Recent investigations into immune mechanisms in cardiovascular health conclusively demonstrate that inflammation independently drives cardiovascular disease, regardless of traditional risk factors (Bartoli-Leonard et al., 2025). This represents a paradigm shift: inflammation isn't merely a marker of disease; it's an active, primary driver. The implications are profound—this means aggressive anti-inflammatory strategies, even in people with normal lipid panels and blood pressure, could yield significant cardiovascular benefits.
Insight 2: Multiple Inflammatory Pathways Operate Simultaneously
Cardiovascular inflammaging doesn't proceed through a single pathway. Rather, multiple interconnected inflammatory mechanisms operate simultaneously: complement activation, pattern recognition receptor signaling, inflammasome activation, and endothelial-derived inflammation all contribute. This multi-pathway nature explains why single-target interventions sometimes fail—blocking one inflammatory pathway may not adequately address the entire inflammatory burden.
Insight 3: Vascular Cells Aren't Passive Victims
Endothelial cells, smooth muscle cells, and cardiac fibroblasts don't passively suffer inflammation's effects. Instead, these cells actively participate in inflammatory amplification, producing and responding to inflammatory mediators in self-perpetuating cycles (Spray et al., 2025). Understanding vascular inflammaging requires recognizing these cells as active participants in disease pathology, not merely passive targets (Zeng et al., 2025).
Insight 4: Sex Differences Matter
Emerging data suggest that inflammaging and its cardiovascular consequences may differ between sexes (Ajoolabady et al., 2024). Estrogen's anti-inflammatory properties mean women enjoy relative cardiovascular protection until menopause, after which inflammatory burden increases. Men often accumulate an inflammatory burden earlier, but both sexes face substantial risk with advancing age.
Therapeutic Perspectives: Moving From Understanding to Action
Anti-inflammatory Pharmacotherapy
Colchicine, historically used for gout, recently demonstrated modest cardiovascular benefits in secondary prevention by targeting inflammasome signalling. This class of anti-inflammatory agents represents a new frontier in cardiovascular medicine. NSAIDs, while anti-inflammatory, carry their own cardiovascular and gastrointestinal risks and don't address underlying inflammaging.
Immunomodulatory Agents
Therapies targeting immune senescence directly—such as CAR-T cell therapies adapted for senescent cell clearance—remain largely experimental but show tremendous promise in preclinical studies
Senolytic Therapies
Drugs specifically designed to eliminate senescent cells (senolytics) are entering clinical trials. Early-stage compounds show potential to reverse some aspects of vascular dysfunction and reduce inflammatory burden
Biological Anti-Inflammatory Interventions
Exercise remains perhaps the most potent anti-inflammatory intervention, reducing multiple inflammatory markers while improving cardiovascular structure and function. Even moderate activity substantially reduces cardiovascular inflammaging manifestations
Caloric restriction and intermittent fasting reduce systemic inflammation and improve vascular function, though long-term adherence remains challenging
Mediterranean-style diets, rich in polyphenols and omega-3 fatty acids, consistently demonstrate anti-inflammatory and cardiovascular benefits through multiple mechanisms.
Emerging Therapeutic Approaches
Targeting the Senescence-Associated Secretory Phenotype
Direct inhibition of SASP factors (particularly IL-6 and TNF-α) represents a rational therapeutic approach These biological agents show promise in reducing inflammaging-related cardiovascular dysfunction.
Gut Microbiota Modulation
The gut microbiota profoundly influences systemic inflammation through multiple mechanisms. Dysbiosis in aging predisposes to increased inflammaging. Therapeutic strategies targeting microbiota composition through prebiotics, probiotics, or targeted antimicrobials show promising preliminary results.
Complement Inhibition
The complement cascade, a key innate immune pathway, becomes dysregulated with aging and hyperactivation contributes to cardiovascular inflammation. Complement inhibitors are entering clinical trials for cardiovascular indications.
Cellular Immunotherapy
Modified immune cells engineered to suppress inflammation while preserving protective immunity represent the frontier of immunotherapeutics . These approaches remain experimental but demonstrate remarkable potential in preclinical models.
Practical Applications: What This Means for You
Assessing Your Inflammatory Burden
While standard cardiovascular risk assessments focus on traditional factors, comprehensive evaluation should include inflammatory biomarkers such as high-sensitivity CRP (hs-CRP), IL-6, TNF-α, lipoprotein(a), and homocysteine. Discussing these with your healthcare provider helps determine whether you might benefit from anti-inflammatory interventions beyond conventional therapy.
Evidence-Based Lifestyle Modifications
Exercise: Aerobic exercise, resistance training, and high-intensity interval training all powerfully reduce cardiovascular inflammaging markers . Aim for 150 minutes weekly of moderate activity or 75 minutes of vigorous activity.
Nutrition: Mediterranean, DASH, and plant-predominant diets consistently reduce inflammatory markers. Emphasize whole grains, fruits, vegetables, legumes, nuts, and fish.
Sleep: Poor sleep quality and insufficient sleep duration increase inflammaging progression. Target 7-9 hours of quality sleep nightly.
Stress Management: Chronic stress amplifies inflammaging. Regular meditation, mindfulness, or other stress-reduction techniques provide measurable cardiovascular benefits.
Smoking Cessation: Smoking is a primary driver of accelerated inflammaging and cardiovascular disease risk
Social Connection: Loneliness and social isolation amplify inflammaging. Maintaining robust social networks provides cardiovascular protection.
When to Consider Pharmacotherapy
Discuss with your cardiologist whether you might benefit from anti-inflammatory medications beyond conventional therapy. Candidates typically include those with elevated inflammatory markers despite conventional risk factor management, secondary prevention patients with established cardiovascular disease, and older adults with high genetic inflammation risk
Frequently Asked Questions
Q1: Can inflammaging be reversed?
Partial reversal appears possible through sustained lifestyle modification and emerging pharmacotherapies. Complete reversal likely isn't feasible, but slowing progression and reducing its cardiovascular impact absolutely is achievable through multi-modal approaches combining exercise, diet, stress reduction, and potentially emerging pharmacotherapies.
Q2: How much does age matter if I control other risk factors?
Age remains a significant independent risk factor. However, aggressive management of modifiable factors substantially reduces age-related risk. An 80-year-old with excellent cardiovascular risk factors faces a lower risk than a 50-year-old with multiple risk factors, though chronological age will always contribute to baseline risk.
Q3: Are anti-inflammatory drugs safe for long-term cardiovascular prevention?
This depends on the specific agent. Traditional NSAIDs carry risks that may outweigh benefits for most people. Emerging agents like colchicine show more favorable safety profiles, but long-term data remain limited. Lifestyle modifications remain the safest, most effective first-line approach.
Q4: Why don't statins reduce all your cardiovascular risk if inflammation is so important?
Statins primarily reduce LDL cholesterol; they have modest anti-inflammatory effects. Residual risk persists due to other factors, including inadequately controlled inflammation. This is why comprehensive, multi-target approaches addressing inflammaging are increasingly recommended.
Q5: Can younger adults benefit from anti-inflammaging strategies?
Absolutely. Preventing inflammaging through lifestyle measures throughout life is vastly easier than reversing it later. Starting exercise, healthy eating, stress management, and adequate sleep in youth prevents the inflammatory accumulation that becomes problematic with age.
Q6: How do inflammatory markers relate to actual cardiovascular events?
Elevated inflammatory markers predict cardiovascular events better than many traditional risk factors alone. However, they're not perfect predictors—some people with high markers remain event-free, while others with low markers experience events. Inflammatory markers provide valuable additional risk stratification when combined with traditional assessment
Key Takeaways: The Bottom Line
Inflammaging is a primary driver of cardiovascular disease in aging not merely an epiphenomenon of age-related changes.
Multiple interconnected mechanisms link aging, immune senescence, and cardiovascular pathology, requiring multi-target therapeutic approaches
Chronic, low-grade systemic inflammation independently predicts cardiovascular events beyond traditional risk factors
Senescent cells accumulate with age and actively fuel cardiovascular inflammaging through their secretory products
Lifestyle modifications remain the most powerful interventions for reducing inflammaging and cardiovascular risk
Emerging pharmacotherapies targeting inflammaging show promise and may enhance prevention and treatment approaches
.
Individual assessment of inflammatory burden using biomarkers can identify high-risk individuals who may benefit from targeted anti-inflammatory intervention
.
Sex-specific differences exist in inflammaging manifestations and may require sex-specific therapeutic approaches
Age-related vascular changes involve active inflammation, not just passive structural deterioration, opening new therapeutic avenues (
Comprehensive cardiovascular prevention must address inflammaging alongside traditional risk factors
Author’s Note
Cardiovascular disease is often framed as a consequence of dyslipidemia, hypertension, and lifestyle excesses. While these factors remain important, contemporary research increasingly demonstrates that they do not fully explain cardiovascular risk—particularly in aging populations. This article was written to highlight cardiovascular inflammaging as a central, biologically driven mechanism that bridges aging, immune dysregulation, and cardiovascular pathology.
The concept of inflammaging reframes cardiovascular disease not merely as a disorder of vessels and lipids, but as a chronic, immune-mediated condition shaped by lifelong inflammatory exposure. By integrating recent advances in immunosenescence, senescent cell biology, endothelial dysfunction, and vascular remodeling, this article aims to translate complex mechanistic science into clinically meaningful insight.
Particular emphasis was placed on evidence from 2024–2025 literature to ensure relevance to current practice. While emerging therapies such as senolytics, immune modulation, and targeted anti-inflammatory pharmacotherapy show promise, this work deliberately underscores the enduring importance of lifestyle interventions—exercise, nutrition, sleep, stress management, and social connection—as foundational anti-inflammaging strategies with the strongest real-world evidence.
This article is intended for clinicians, researchers, and scientifically engaged readers seeking a deeper understanding of why cardiovascular risk rises with age even in the absence of traditional risk factors. It also serves as a call to broaden cardiovascular prevention beyond cholesterol and blood pressure, toward a more integrative model that addresses immune aging and chronic inflammation as modifiable drivers of disease.
Ongoing research will undoubtedly refine biomarkers, clarify patient selection for anti-inflammatory therapies, and determine the long-term safety of novel interventions. Until then, recognizing and addressing inflammaging represents one of the most promising frontiers in preventive cardiology and healthy aging.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Statins, Side Effects, and the Placebo Problem: What Recent Research Really Shows | DR T S DIDWAL
The Cholesterol Paradox: When Lower Numbers Don’t Mean Lower Heart Risk | DR T S DIDWAL
hsCRP in Cardiovascular Disease: Should It Be Measured for Risk Assessment in 2026? | DR T S DIDWAL
Your Body Fat Is an Endocrine Organ—And Its Hormones Shape Your Heart Health | DR T S DIDWAL
hsCRP Explained: What Inflammation Means for Your Heart | DR T S DIDWAL
What’s New in the 2025 Blood Pressure Guidelines? A Complete Scientific Breakdown | DR T S DIDWAL
Low-Fat vs. Low-Carb: Which Diet is Best for Weight Loss? | DR T S DIDWAL
5 Steps to Reverse Metabolic Syndrome: Diet, Habit, & Lifestyle Plan | DR T S DIDWAL
The Role of Cholesterol in Health and Disease: Beyond the "Bad" Label | DR T S DIDWAL
Lowering Cholesterol with Food: 4 Phases of Dietary Dyslipidemia Treatment | DR T S DIDWAL
The Best Dietary Fat Balance for Insulin Sensitivity, Inflammation, and Longevity | DR T S DIDWAL
References
Ajoolabady, A., Pratico, D., Tang, D., Zhou, S., Franceschi, C., & Ren, J. (2024). Immunosenescence and inflammaging: Mechanisms and role in diseases. Ageing Research Reviews, 101, 102540. https://doi.org/10.1016/j.arr.2024.102540
Bartoli-Leonard, F., Clark, D. E., Singhal, N. S., & Nguyen, P. K. (2025). Unmasking the hidden burden: Inflammation and cardiovascular disease JAHA spotlight on the role of the immune system in cardiovascular health and disease. Journal of the American Heart Association, e047298. Advance online publication. https://doi.org/10.1161/JAHA.125.047298
Spray, L., Richardson, G., Haendeler, J., Altschmied, J., Rumampouw, V., Wallis, S. B., Georgiopoulos, G., White, S., Unsworth, A., Stellos, K., Tual-Chalot, S., & Spyridopoulos, I. (2025). Cardiovascular inflammaging: Mechanisms, consequences, and therapeutic perspectives. Cell Reports Medicine,c 6(9), 102264. https://doi.org/10.1016/j.xcrm.2025.102264
Zeng, Y., Buonfiglio, F., Li, J., Pfeiffer, N., & Gericke, A. (2025). Mechanisms underlying vascular inflammaging: current insights and potential treatment approaches. Aging and Disease, 16(4), 1889. https://doi.org/10.14336/ad.2024.0922