Your Body Fat Is an Endocrine Organ—And Its Hormones Shape Your Heart Health
Is your body fat an endocrine organ? Learn how "sick fat" triggers metabolic inflammation and heart disease, and discover emerging biomarkers for early cardiovascular detection.
OBESITYHEART
Dr. T.S. Didwal, M.D.(Internal Medicine)
1/14/202611 min read
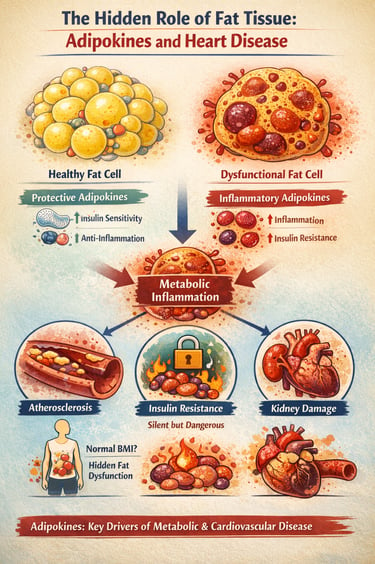
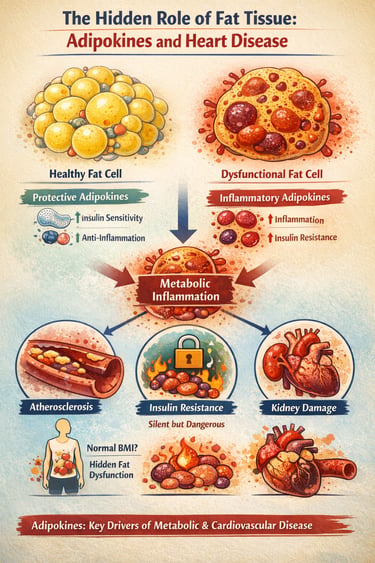
What if the very tissue we often blame for weight gain is actually sending powerful chemical messages that shape your metabolism, inflammation, and even your heart health? Modern research reveals that adipose tissue is not passive fat storage—it is a dynamic endocrine organ, constantly releasing hormones and inflammatory molecules called adipokines that influence every major metabolic pathway (Tilg et al., 2025).
In a healthy state, adipose tissue produces protective adipokines like adiponectin, which enhance insulin sensitivity and reduce inflammation. But when fat cells enlarge or become dysfunctional, this balance shifts dramatically. The body begins to release harmful inflammatory molecules such as TNF-α and IL-6, creating a state of chronic, low-grade metabolic inflammation that silently drives insulin resistance, vascular injury, and early atherosclerosis (Gao et al., 2025; He et al., 2026).
Even individuals with normal body weight can develop this hidden dysfunction, making traditional measurements like BMI increasingly inadequate for assessing metabolic risk (Ramos-Zavala et al., 2025). The result is a growing recognition among clinicians that cardiometabolic disease begins not in the bloodstream—but deep within dysfunctional fat tissue.
Understanding this adipokine imbalance may be the key to predicting, preventing, and reversing metabolic and cardiovascular disease in the years ahead.
This comprehensive guide explores the cutting-edge research on adipokines, their role in metabolic inflammation, and their implications for preventing and managing cardiovascular disease. We'll synthesize findings from the latest 2025-2026 studies to help you understand how fat tissue dysfunction contributes to disease and what emerging therapies may offer hope.
Clinical pearls
1. The "Quality over Quantity" Rule
In the world of fat tissue, function matters more than volume. You can be at a "normal" weight but still have "sick" fat (adipose tissue dysfunction) that secretes inflammatory markers. Conversely, healthy fat cells that are small and insulin-sensitive act as a protective endocrine organ.
The Pearl: Don't just chase a number on the scale; focus on metabolic markers like blood sugar and waist circumference, which are better indicators of how your fat is "behaving."
2. Adiponectin: Your Internal "Fire Extinguisher"
Think of Adiponectin as your body’s natural anti-inflammatory. In healthy states, levels are high, keeping your arteries flexible and your insulin working well. As fat cells enlarge (hypertrophy), they "turn off" the production of this protector.
The Pearl: Exercise is one of the most potent ways to boost Adiponectin. It’s essentially a way to "re-program" your fat cells to start producing protective signals again.
3. The "Silent" Nature of Metabolic Inflammation
Unlike a swollen ankle or a sore throat, metabolic inflammation (driven by TNF-α and IL-6) is low-grade and invisible. It doesn't cause pain, but it acts like "sand in the gears" of your cardiovascular system, slowly scratching the lining of your arteries (endothelial dysfunction).
The Pearl: Because you can’t "feel" metabolic inflammation, regular screening of high-sensitivity C-reactive protein (hs-CRP) can provide a "weather report" for your internal inflammatory climate.
4. Adipocyte Hypertrophy and "Crosstalk"
When fat cells get too large, they become stressed and begin to leak inflammatory signals. These signals don't stay in the fat; they travel through the blood to talk to your heart, liver, and kidneys, telling them to become resistant to insulin and more prone to scarring.
The Pearl: Weight distribution is a key clue. Fat stored around the midsection (visceral fat) is much more likely to be "loud" and inflammatory than fat stored under the skin on the hips or legs.
5. The "Ratios" are the Future of Risk
Modern medicine is moving away from looking at just one number (like total cholesterol). We now look at the Leptin-to-Adiponectin ratio. Leptin signals "fullness" but can become inflammatory when too high, while Adiponectin protects.
The Pearl: A high ratio means your metabolic "brakes" (Adiponectin) are failing while your "gas pedal" for inflammation (Leptin) is stuck to the floor. Future heart health is about restoring that balance.
Adipokines, Metabolic Inflammation, and Cardiovascular Health: Understanding the Hidden Connection Between Fat Tissue and Heart Disease
Part 1: Understanding Adipokines and Their Functions
Adipokines are secreted proteins and bioactive molecules produced primarily by adipocytes (fat cells) and immune cells within adipose tissue. These powerful signaling molecules regulate energy metabolism, immune function, and vascular health—influencing nearly every system in the body.
The most well-studied adipokines include:
Adiponectin: Often called the "good" adipokine, it promotes insulin sensitivity and anti-inflammatory responses
Leptin: Regulates appetite and energy expenditure
TNF-α (Tumor Necrosis Factor-alpha): A pro-inflammatory cytokine elevated in obesity
IL-6 (Interleukin-6): Another inflammatory mediator linked to metabolic disease
Netrin-1: An emerging adipokine with complex metabolic effects
According to Tilg et al. (2025), adipokines function as "masterminds of metabolic inflammation," orchestrating the immune response and metabolic homeostasis. These molecules don't simply respond to metabolic changes—they actively drive them, making them central to understanding modern chronic disease.
The Dual Nature of Adipokines: Protective and Pathogenic
One of the most fascinating discoveries in recent adipokine research is their dual functionality. The same adipose tissue can produce both protective and inflammatory molecules, and the balance between them determines metabolic health.
Ramos-Zavala et al. (2025) demonstrated opposing profiles of two critical adipokines: Netrin-1 and adiponectin. In their comprehensive study on metabolic inflammation and insulin resistance, they found that while adiponectin exerts protective, anti-inflammatory effects, Netrin-1 displays a more complex profile. The opposing patterns of these molecules suggest that metabolic disease emerges not from a single "bad" molecule, but from a dysregulated balance between protective and harmful adipokines.
This finding has profound implications: therapeutic approaches targeting a single adipokine may be less effective than strategies promoting overall adipokine balance.
Part 2: Adipose Tissue Dysfunction and Metabolic Dysregulation
The Pathophysiology of Adipose Tissue Dysfunction
When adipose tissue becomes dysfunctional, its ability to produce appropriate adipokine profiles deteriorates. This condition, termed adipose tissue dysfunction, represents a fundamental breakdown in the tissue's endocrine and metabolic functions.
Bou Matar et al. (2025) published a landmark study in Frontiers in Endocrinology examining how adipose tissue dysfunction disrupts metabolic homeostasis. Their research identified several key mechanisms linking fat dysregulation to disease:
Impaired adipokine secretion patterns: Dysfunctional adipose tissue fails to suppress inflammatory adipokines and reduce protective ones
Mitochondrial dysfunction: Energy production within fat cells becomes inefficient
Increased oxidative stress: Reactive oxygen species accumulate, damaging cells
Altered immune cell infiltration: Macrophages and other immune cells invade the tissue, promoting chronic inflammation
Metabolic inflexibility: Fat tissue loses the ability to switch between different metabolic states
The consequences ripple throughout the body: impaired glucose metabolism, worsening insulin resistance, increased cardiovascular inflammation, and accelerated progression toward metabolic syndrome and heart disease.
Adipocyte Hypertrophy and Inflammatory Dysfunction
One particularly damaging form of adipose dysfunction occurs when fat cells enlarge abnormally—a condition called adipocyte hypertrophy. Gao et al. (2025) investigated this phenomenon in their study on disrupted adipokine secretion and inflammatory responses in human adipocyte hypertrophy.
Their findings revealed that hypertrophied adipocytes—enlarged fat cells—exhibit fundamentally altered secretion patterns. Rather than producing balanced levels of protective and inflammatory molecules, enlarged cells shift toward a pro-inflammatory profile. This creates a vicious cycle:
Enlarged adipocytes produce more TNF-α and IL-6
These inflammatory molecules trigger further metabolic dysfunction
The tissue becomes increasingly resistant to insulin and other regulatory signals
The dysfunction spreads to neighboring cells, amplifying the problem
This research suggests that adipocyte size itself serves as a marker of dysfunction—one reason why weight distribution matters as much as total body weight for metabolic health.
Part 3: The Adipokine-Inflammation Connection in Metabolic Disease
Metabolic Inflammation: The Silent Killer Driving Chronic Disease
Metabolic inflammation differs subtly but critically from acute inflammation. While acute inflammation is a beneficial, short-lived immune response to injury or infection, metabolic inflammation is chronic, systemic, and often occurs without obvious infection or injury. It's the "low-grade," persistent activation of immune pathways that drives metabolic disease.
He et al. (2026) published a major study in Pathology Research and Practice documenting adipokine-mediated crosstalk between metabolic dysregulation and inflammatory pathways. Their research demonstrated that adipokines don't simply trigger inflammation—they actively coordinate communication between metabolic and immune systems.
Key findings include:
Adipokines activate pattern recognition receptors on immune cells, priming them for inflammatory responses
Metabolic byproducts (like free fatty acids and lipopolysaccharides) activate these same pathways
Feedback loops between adipokines and immune cytokines amplify inflammatory signals
Tissue-specific effects: Adipokine signaling differs between liver, muscle, pancreas, and other organs
This integrated view explains why simple interventions often fail: you cannot address metabolic inflammation by targeting a single pathway. Instead, therapeutic approaches must consider the complex, interconnected nature of adipokine-immune-metabolic crosstalk.
The Role of Adipokines in Diabetic Kidney Disease
One particularly important clinical manifestation of adipokine dysregulation involves the kidneys. Yang et al. (2026) investigated adipokine networks in diabetic kidney disease, revealing mechanistic insights and therapeutic opportunities.
Their research demonstrates that:
Adiponectin deficiency contributes to diabetic kidney disease progression
Inflammatory adipokines activate kidney resident immune cells, promoting glomerular injury
Lipotoxicity (toxicity from excessive lipid accumulation) damages the kidney's filtering structures
Adipokine signaling can be therapeutically targeted to slow or reverse kidney damage
This work illustrates how understanding adipokine pathophysiology translates into clinical insights for specific disease prevention and treatment.
Part 4: Cardiovascular Implications of Adipokine Dysfunction
Adipokines and Cardiovascular Risk Stratification
The connection between adipose tissue dysfunction and cardiovascular disease extends far beyond simple cholesterol levels or blood pressure. Modern cardiovascular biomarkers research increasingly recognizes adipokines as critical indicators of heart disease risk.
Netala et al. (2025) published a comprehensive review in the International Journal of Molecular Sciences on cardiovascular biomarkers as tools for precision diagnosis and prognosis. Their analysis highlights emerging adipokine biomarkers that improve our ability to identify high-risk patients before they experience heart attacks or strokes.
Key biomarkers discussed include:
Adiponectin levels: Low adiponectin predicts increased cardiovascular risk
Leptin/adiponectin ratio: An imbalance indicates metabolic dysfunction and vascular disease risk
Inflammatory adipokines (TNF-α, IL-6): Elevated levels correlate with atherosclerotic burden
Novel adipokines: Emerging molecules like Netrin-1 may refine risk prediction
These precision biomarkers enable stratification of patients into risk categories, allowing personalized prevention and treatment strategies.
Vascular Dysfunction and Adipokine-Mediated Inflammation
Beyond metabolic effects, adipokines directly influence vascular function. Kishimoto & Higashi (2025) published a major update in Hypertension Research on recent advances in vascular and cardiovascular research, emphasizing the critical role of adipokine-mediated pathways.
Their findings detail how dysfunctional adipose tissue triggers:
Endothelial dysfunction: The inner lining of blood vessels loses its ability to regulate blood flow and control inflammation
Arterial stiffness: Blood vessels become less elastic, increasing blood pressure and cardiac workload
Atherosclerotic plaque formation: Inflammatory adipokines promote the accumulation of lipids and immune cells within arterial walls
Increased thrombotic risk: Blood clotting tendency increases, raising heart attack and stroke risk
The mechanistic link is clear: adipokine dysregulation initiates a cascade of vascular pathology that culminates in clinical cardiovascular events.
Advanced Inflammatory Markers as Predictive Tools
Koo et al. (2025) conducted a systematic review of advanced inflammatory markers as predictors of cardiovascular disease, synthesizing evidence from multiple studies on how biomarkers improve cardiovascular risk assessment.
Their comprehensive analysis identified adipokine-related inflammatory markers among the most promising for predicting future cardiovascular events:
High-sensitivity C-reactive protein (hs-CRP): Often elevated in adipose dysfunction
Interleukin-6 (IL-6): Produced by dysfunctional adipose tissue; predicts cardiovascular outcomes
Tumor necrosis factor-alpha (TNF-α): Correlates with arterial inflammation and plaque progression
Adipokine ratios: The balance between protective and inflammatory molecules predicts risk better than single markers
Early Detection and Risk Stratification Advances
Nazir et al. (2025) reviewed advancements in biomarkers for early detection and risk stratification of cardiovascular disease, emphasizing the emerging role of adipokine profiling. Their literature review highlights how modern laboratory techniques enable comprehensive adipokine assessment that was impossible just years ago.
Clinical applications include:
Screening asymptomatic individuals with metabolic risk factors
Monitoring progression of cardiovascular disease in known patients
Assessing response to lifestyle and pharmacological interventions
Identifying resistant cases requiring more aggressive treatment
Part 5: Therapeutic Implications and Future Directions
Current and Emerging Therapeutic Approaches
Understanding adipokine pathophysiology opens new therapeutic avenues. Current approaches include:
Lifestyle Interventions: Regular physical activity and dietary modification remain the most effective ways to restore adipose tissue function and normalize adipokine profiles. Even moderate weight loss can significantly improve adiponectin levels and reduce inflammatory adipokines.
Pharmacological Therapies: Several drug classes target adipokine pathways:
GLP-1 receptor agonists: Improve adipose function and reduce inflammation
SGLT2 inhibitors: Protect against metabolic inflammation independent of weight loss
Thiazolidinediones: Directly improve adipose tissue insulin sensitivity
Emerging Approaches: Research on direct adipokine supplementation (adiponectin) and Netrin-1 modulation shows promise in preclinical and early clinical studies.
Key Takeaways: What You Need to Know
Adipose tissue is an active endocrine organ producing powerful signaling molecules (adipokines) that regulate metabolism, inflammation, and cardiovascular health
Adipokine dysbalance, not obesity per se, drives metabolic disease. Protective adipokines (like adiponectin) are reduced while inflammatory ones (TNF-α, IL-6) are elevated
Metabolic inflammation is a chronic, systemic immune activation linked to adipose dysfunction. It represents a key mechanism connecting fat tissue to cardiovascular disease
Adipocyte hypertrophy (enlarged fat cells) specifically triggers pro-inflammatory adipokine secretion patterns, creating a vicious cycle of worsening dysfunction
Novel adipokines like Netrin-1 display complex opposing effects, suggesting that future therapies must address the balance of multiple molecules rather than single targets
Cardiovascular biomarkers increasingly incorporate adipokine assessment for better risk prediction, early detection, and personalized treatment strategies
Therapeutic interventions targeting adipose function (lifestyle modification, emerging pharmacotherapy) can restore normal adipokine profiles and prevent cardiovascular complications
Frequently Asked Questions
Q: Can I have normal weight but still have adipose tissue dysfunction? A: Yes. Metabolically obese normal-weight (MONW) individuals can have dysfunctional adipose tissue. What matters is the metabolic quality of fat tissue, not just quantity.
Q: How quickly can adipokine profiles improve with lifestyle changes? A: Adiponectin levels and inflammatory markers can improve within weeks to months of sustained physical activity and dietary improvement, though complete restoration takes longer.
Q: Are adipokine biomarkers currently available in clinical practice? A: Some adipokines (particularly adiponectin and inflammatory cytokines) are measurable in specialized labs. However, comprehensive adipokine profiling isn't yet standard clinical practice but is emerging in research settings.
Q: Is adipose tissue dysfunction reversible? A: In most cases, yes—particularly in earlier stages. Restoration of normal adipokine patterns can occur with sustained lifestyle modification or appropriate pharmacotherapy.
Q: What's the connection between adipokines and insulin resistance? A: Dysfunctional adipose tissue produces fewer protective molecules (adiponectin) while increasing inflammatory adipokines. These changes directly impair insulin signaling in liver, muscle, and adipose tissue itself.
Q: Can diet influence adipokine production? A: Absolutely. Diets rich in whole grains, fiber, and unsaturated fats promote healthy adipokine profiles, while ultra-processed foods and excess sugar worsen adipose dysfunction.
Author’s Note
As a physician and researcher deeply involved in the study of metabolic disease, I wrote this article to bridge a critical knowledge gap that exists in both clinical practice and public understanding. For years, fat tissue was dismissed as a passive storage organ—until modern science revealed its true identity: an active endocrine system capable of shaping our metabolic destiny. The emerging evidence on adipokines, metabolic inflammation, and cardiovascular risk is not merely academic; it has profound implications for how we diagnose, prevent, and treat chronic disease.
My goal in preparing this piece was to translate complex, cutting-edge findings from 2025–2026 into clear, actionable insights for clinicians, researchers, and health-conscious readers alike. Every study cited represents a step forward in our understanding of how adipose tissue dysfunction silently drives insulin resistance, vascular injury, and long-term cardiovascular complications. By highlighting the interplay between adipokines, immune activation, and cardiometabolic pathways, I hope to encourage a more nuanced, systems-based approach to patient care.
Ultimately, this article is an invitation—to rethink how we view body fat, to embrace biomarker-driven precision medicine, and to adopt lifestyle and therapeutic strategies that restore adipose health. Your metabolic health begins with understanding the signals your fat tissue sends.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Activate Your Brown Fat: A New Pathway to Longevity and Metabolic Health | DR T S DIDWAL
Leptin vs. Adiponectin: How Your Fat Hormones Control Weight and Metabolic Health | DR T S DIDWAL
Lower Blood Pressure Naturally: Evidence-Based Exercise Guide for Metabolic Syndrome | DR T S DIDWAL
Movement Snacks: How VILPA Delivers Max Health Benefits in Minutes | DR T S DIDWAL
The BMI Paradox: Why "Normal Weight" People Still Get High Blood Pressure | DR T S DIDWAL
How Insulin Resistance Accelerates Cardiovascular Aging | DR T S DIDWAL
References
Bou Matar, D., Zhra, M., Nassar, W. K., Altemyatt, H., Naureen, A., Abotouk, N., Elahi, M. A., & Aljada, A. (2025). Adipose tissue dysfunction disrupts metabolic homeostasis: Mechanisms linking fat dysregulation to disease. Frontiers in Endocrinology, 16, 1592683. https://doi.org/10.3389/fendo.2025.1592683
Gao, D., Bing, C., & Griffiths, H. R. (2025). Disrupted adipokine secretion and inflammatory responses in human adipocyte hypertrophy. Adipocyte, 14(1). https://doi.org/10.1080/21623945.2025.2485927
He, Z., Zhao, L., Liu, J., Zheng, W., Gong, C., Guo, L., & Liang, T. (2026). Adipokine-mediated crosstalk between metabolic dysregulation and inflammatory pathways. Pathology Research and Practice, 278, 156312. https://doi.org/10.1016/j.prp.2025.156312
Kishimoto, S., & Higashi, Y. (2026). Recent advances and emerging perspectives in vascular and cardiovascular research: A 2025 update. Hypertension Research, 49, 1–12. https://doi.org/10.1038/s41440-025-02540-1
Koo, T. H., Leong, X. B., & Mohamed, M. (2025). Systematic review of advanced inflammatory markers as predictors of cardiovascular diseases. Research in Cardiovascular Medicine, 14(1), 8–14. https://doi.org/10.4103/rcm.rcm_35_24
Nazir, A., Nazir, A., Afzaal, U., Aman, S., Sadiq, S. U. R., Akah, O. Z., Jamal, M. S. W., & Hassan, S. Z. (2025). Advancements in biomarkers for early detection and risk stratification of cardiovascular diseases—A literature review. Health Science Reports, 8(5), e70878. https://doi.org/10.1002/hsr2.70878
Netala, V. R., Hou, T., Wang, Y., Zhang, Z., & Teertam, S. K. (2025). Cardiovascular biomarkers: Tools for precision diagnosis and prognosis. International Journal of Molecular Sciences, 26(7), 3218. https://doi.org/10.3390/ijms26073218
Ramos-Zavala, M. G., García-Galindo, J. J., Beltrán-Ramírez, A., Balleza-Alejandri, L. R., Peña-Durán, E., Huerta-Huerta, A., Rubio-Arellano, E. D., López-Murillo, L. D., Pascoe-Gonzalez, S., Campos-Bayardo, T. I., & Suárez-Rico, D. O. (2025). Opposing profiles of Netrin-1 and adiponectin in metabolic inflammation and insulin resistance. Frontiers in Pharmacology, 16, 1632956. https://doi.org/10.3389/fphar.2025.1632956
Tilg, H., Ianiro, G., Gasbarrini, A., & Adolph, T. E. (2025). Adipokines: Masterminds of metabolic inflammation. Nature Reviews Immunology, 25(4), 250–265. https://doi.org/10.1038/s41577-024-01103-8
Yang, K., Fang, Y., He, J., et al. (2026). Adipokine networks in diabetic kidney disease: Mechanistic insights and therapeutic implications. Lipids in Health and Disease. https://doi.org/10.1186/s12944-025-02851-9