Statins, Side Effects, and the Placebo Problem: What Recent Research Really Shows
Explore what modern research reveals about statins, side effects, and the placebo effect—and how to separate real risk from perceived harm.
HEART
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/8/202611 min read
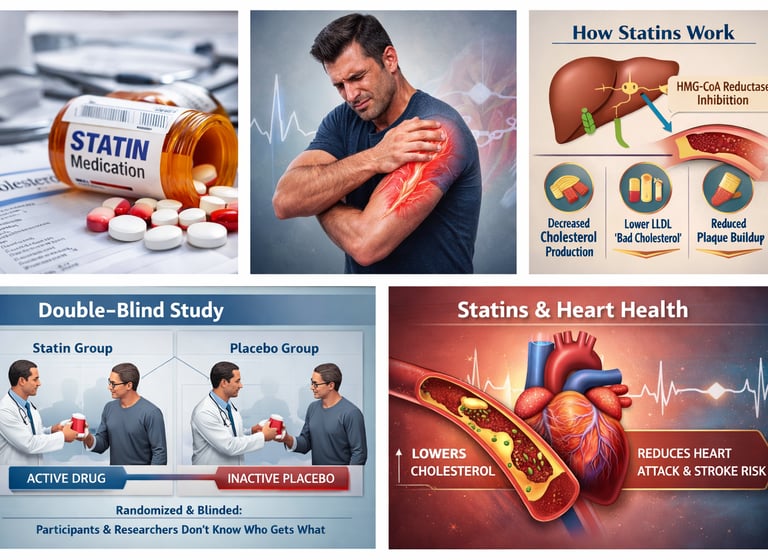
For decades, statins have occupied a paradoxical place in modern medicine. They are among the most rigorously studied and widely prescribed drugs in history—yet they are also among the most feared. Patients routinely report muscle pain, fatigue, and weakness shortly after starting therapy, while clinicians struggle to determine whether these symptoms are truly caused by the drug or simply coincidental. This tension has fueled public skepticism, sensational headlines, and a growing mistrust of statin therapy itself.
Recent research, however, is forcing a long-overdue recalibration of this debate. Large-scale analyses of double-blind randomized controlled trials—the gold standard of clinical evidence—now suggest that many adverse effects listed on statin product labels occur at similar rates in placebo groups, raising critical questions about how side effects are attributed and communicated (Reith et al., 2026). At the same time, mechanistic studies have finally clarified why a subset of patients genuinely experiences statin-related muscle symptoms, identifying distinct biochemical pathways that confer individual susceptibility (University of British Columbia, 2026).
Complicating matters further, emerging evidence challenges the long-standing assumption that “lower LDL at any cost” is always the optimal strategy. Precision-based approaches to statin dosing may reduce adverse effects without sacrificing cardiovascular protection, particularly in patients with stable coronary disease (Dimmitt et al., 2026). Meanwhile, authoritative voices in preventive cardiology are urging greater caution when prescribing statins for primary prevention in low- to intermediate-risk individuals, where absolute benefit may be modest (Demasi et al., 2025).
Together, these findings do not indict statins—but they do expose a deeper truth: the problem has never been the drug itself. It has been our failure to distinguish correlation from causation, population averages from individual biology, and risk reduction from risk communication. Understanding this distinction is no longer optional—it is essential for evidence-based cardiovascular care.
Clinical pearls
1. The "Nocebo" Nuance
Science shows that the anticipation of side effects can be as powerful as the drug itself. In double-blind trials, many patients reported muscle pain while unknowingly taking a sugar pill. While your pain is real, it may sometimes be a "nocebo" response—a physical reaction to the fear of the medication rather than the chemical itself.
2. The Mechanism is Mapped
For years, doctors couldn't explain why statins caused muscle aches, leading some to dismiss patient complaints. We now know specific biochemical pathways in muscle tissue can be disrupted in susceptible individuals. If you feel pain, it’s not "all in your head"; it may be a measurable cellular response unique to your biology.
3. Absolute Risk vs. Relative Numbers
A "30% reduction in heart attack risk" sounds massive, but it’s a relative number. If your starting risk is only 1%, a 30% reduction brings you to 0.7%. Clinical wisdom suggests focusing on your absolute risk—the actual "chance in a hundred" of an event—to decide if daily medication is worth the potential for side effects.
4. Precision Dosing: Less can be More
The "fire and forget" method of prescribing the highest possible dose is being replaced by Precision Medicine. For many, a moderate dose provides the bulk of the life-saving benefits with a significantly lower risk of "hitting the wall" with side effects. More medication does not always equal more protection.
5. The Genetic "Speed Limit"
Your liver uses specific enzymes (like the CYP450 system) to process statins. Think of these as your body’s internal speed limit. If your genetics make you a "slow metabolizer," the drug stays in your system longer, increasing the risk of side effects. Genetic testing or a simple dose adjustment can help "tune" the drug to your body's speed.
6. Prevention is a Spectrum
Statins are a "gold standard" for secondary prevention (people who have already had a heart event). However, for primary prevention (healthy people with high numbers), the evidence is more nuanced. In these cases, the "clinical pearl" is that lifestyle—movement, metabolic health, and nutrition—remains the most potent "medication" with the fewest side effects.
What Are Statins? A Quick Primer
Before diving into the research, let's establish what we're talking about. Statins are a class of medications that lower cholesterol levels by blocking an enzyme in the liver. They're prescribed for both primary prevention (preventing a first heart attack in people without prior heart disease) and secondary prevention (preventing another event in those who've already had one).
The most commonly prescribed statins include atorvastatin (Lipitor), simvastatin (Zocor), and rosuvastatin (Crestor). Over 40 million Americans take statins daily, making them one of the most prescribed drug classes globally.
The Problem: Are Product Labels Overstating Statin Adverse Effects?
A major 2026 study published in The Lancet has sparked a serious conversation about how statin adverse effects are reported on medication labels. According to Reith et al. (2026), researchers conducted a comprehensive meta-analysis of double-blind randomised controlled trials to assess adverse effects attributed to statin therapy in product labels.
Key Takeaway: This landmark meta-analysis examined whether the adverse effects listed on statin packaging actually match what we see in rigorous clinical trials. The distinction is crucial—there's a significant difference between events that occur in trial participants who are taking statins and events that are actually caused by statins.
Why This Matters
The research found substantial discrepancies between what product labels claim and what actual double-blind, randomised controlled trials demonstrate. When patients know they're taking a medication (or believe they might be), the placebo effect kicks in—people may attribute normal aches, pains, and fatigue to their medication even when those symptoms have nothing to do with the drug itself.
By examining only double-blind studies where neither patients nor researchers knew who received statins versus placebos, scientists could isolate the true statin adverse effects from coincidental symptoms.
Statin Muscle Pain: Finally Explained
One of the most bothersome side effects patients report is statin muscle pain—or myalgia, as doctors call it. For years, the mechanism remained mysterious. In January 2026, researchers at the University of British Columbia made a breakthrough discovery.
According to the University of British Columbia (2026) research, scientists finally explained statin muscle pain through research identifying the cellular mechanisms behind this phenomenon. Rather than being a psychosomatic effect or a rare adverse event, the research revealed specific biochemical pathways through which certain individuals develop muscle symptoms in response to statin therapy.
Key Takeaway: Understanding why some people experience statin muscle pain while others don't is the first step toward better patient selection and dosing strategies. This research validates the experience of patients who genuinely do experience muscle discomfort while also helping distinguish this subset from the much larger group experiencing symptoms unrelated to their medication.
The UBC research suggests that statin muscle pain isn't simply "all in your head." For susceptible individuals, statins genuinely do cause measurable changes in muscle tissue that can result in pain and weakness. However, this doesn't mean that everyone taking statins will experience these symptoms—genetic factors, dosage, and individual biochemistry all play roles.
Rethinking Statin Dosage: A New Paradigm for Coronary Disease
While higher doses of statins are more effective at lowering cholesterol, more isn't always better when it comes to patient outcomes. A 2026 study published in Pharmacology Research & Perspectives challenges conventional dosing wisdom.
Dimmitt et al. (2026) in their research on "Rethinking statin dosage in coronary disease" argue for a reconsideration of how statin doses are determined and prescribed to patients with coronary disease.
Key Takeaway: Not every patient with coronary disease needs maximum-dose statin therapy. A personalized medicine approach—tailoring statin dosage to individual risk factors, genetics, and tolerance—may provide better outcomes with fewer adverse effects.
The standard approach has been "lower your LDL cholesterol as much as possible," leading doctors to prescribe high-dose statins to most patients. However, the Dimmitt research suggests this one-size-fits-all approach may be creating unnecessary side effects in patients who would do just fine on moderate doses.
Factors to consider for optimal statin dosing:
Genetic variations in how your body metabolizes statins (pharmacogenetics)
Age and overall health status
Existing muscle problems or family history of statin sensitivity
Concomitant medications that might interact with statins
Absolute cardiovascular risk rather than just LDL numbers
Primary Prevention vs. Secondary Prevention: Different Risks, Different Decisions
Perhaps the most controversial use of statins is in primary prevention—prescribing them to people who've never had a heart attack or stroke but have high cholesterol or other risk factors. A 2025 perspective piece by Demasi et al. (2025) in JAMA raises important questions about this practice.
Key Takeaway: The evidence for statin primary prevention in all patients is less robust than the evidence for secondary prevention (in those with established heart disease). For some people—particularly those at genuinely high risk—statins can be life-saving. For others with borderline risk factors, the benefit may not outweigh the burden of daily medication and potential adverse effects.
Understanding Your Personal Risk
The decision to take a statin for primary prevention should be individualized. Consider:
Your absolute cardiovascular risk over the next 10 years (not just your cholesterol number)
Your family history of early heart disease
Your ability to tolerate the medication and potential side effects
Other lifestyle factors like diet, exercise, and stress management that can reduce risk without medication
The Evidence: What Double-Blind Randomised Controlled Trials Actually Show
One of the most important insights from these studies is understanding what "adverse effect" actually means. In clinical trials:
Reported events = everything a participant experiences while taking the drug
True adverse effects = events that occur significantly more often in the drug group than in the placebo group
Incidental events = things that happen to coincide with taking the drug but aren't caused by it
Consider this: If 10 people take a placebo for a year, some will develop a headache. If 10 people take a statin for a year, some will also develop a headache—not necessarily because of the statin, but because headaches happen. Double-blind randomised controlled trials help us distinguish the signal from the noise.
The Role of Product Labels in Patient Perception
Product labels are required by law to list all adverse effects that occurred more frequently in drug groups than placebo groups, even if the difference is small. This is done for safety reasons, but it can create a perception that these side effects are common and inevitable.
The Reith meta-analysis specifically examined this labeling challenge, asking: Are patients getting an accurate picture of their actual risk?
FAQs: Your Questions About Statins Answered
Q1: If I take a statin, will I definitely get muscle pain?
A: No. While statin muscle pain is a known possible adverse effect, it doesn't occur in everyone. Studies show that the incidence of statin-related muscle symptoms varies widely depending on the statin, dose, individual factors, and—critically—whether patients know they're taking the medication (placebo effect). Many people take statins for years without experiencing muscle symptoms.
Q2: Are the side effects listed on statin labels overblown?
A: The Reith meta-analysis suggests that product labels may include adverse effects that occur at similar rates in both statin users and placebo users. This doesn't mean the labels are wrong—it means they're listing all events that occurred, not necessarily all events caused by statins. Understanding this distinction helps you interpret the information more accurately.
Q3: Should everyone take a statin?
A: No. The Demasi research emphasizes that statin primary prevention decisions should be individualized based on absolute cardiovascular risk, not just cholesterol numbers. For people with established coronary disease, statins are often beneficial. For others, lifestyle modifications might be the first step.
Q4: Can I take a lower dose of statin?
A: Possibly. The Dimmitt research on rethinking statin dosage suggests that some patients may do well on moderate doses rather than maximum doses. However, this is a decision to make with your doctor based on your individual situation, cardiovascular risk, and tolerance.
Q5: How common is statin-induced muscle damage?
A: True statin-related muscle injury is relatively rare. Minor muscle aches occur in some patients, but severe muscle damage (rhabdomyolysis) is extremely uncommon—occurring in fewer than 3 per 100,000 users annually. The University of British Columbia research helps explain why some people are susceptible while others aren't.
Q6: Should I stop taking my statin because of side effects?
A: Don't stop without talking to your doctor. If you're experiencing symptoms you believe might be statin adverse effects, it's important to discuss them. Your doctor might adjust your dose, switch you to a different statin, or investigate other causes of your symptoms. For people with established heart disease, the benefits of statins typically outweigh the risks, but this must be individualized.
Q7: How do genetic factors affect statin response?
A: Your genes influence how quickly your body breaks down statins, how you respond to their effects, and whether you're prone to statin adverse effects. The Dimmitt research emphasizes that personalized medicine approaches considering pharmacogenetics could lead to better outcomes with fewer side effects.
Key Takeaways: What You Need to Know
For Patients Currently Taking Statins
Your experience is valid: If you're experiencing symptoms, don't dismiss them as imagination, even if your doctor initially attributes them elsewhere
Individual variation matters: Not everyone experiences statin adverse effects the same way
Your dose might not be optimal: Recent research suggests a precision medicine approach to dosing rather than a maximum-dose-for-everyone
Reporting matters: If you experience new symptoms after starting a statin, report them to your doctor so they can determine if an adjustment is needed
For Those Considering Statin Therapy
Know your personal risk: Primary prevention decisions should be based on your absolute cardiovascular risk, not just cholesterol numbers
Understand the evidence: Double-blind randomised controlled trials provide the most reliable data on true statin adverse effects
Have the conversation: Discuss benefits AND risks specific to your situation with your healthcare provider
Consider lifestyle first: For many people without established heart disease, diet and exercise modifications may be tried first
For Healthcare Providers
Product labels vs. causation: Be aware that adverse effects listed on product labels may include coincidental events not actually caused by statins
Personalize your approach: The Dimmitt research supports reconsidering one-size-fits-all dosing strategies
Validate patient experience: The UBC research on statin muscle pain mechanisms validates that some patients genuinely experience drug-related symptoms
Educate patients: Help them understand the difference between reported events and causal adverse effects
The Bottom Line: Evidence-Based Decisions
The latest research on statin adverse effects tells us several important things:
Product labels may overstate actual causation: Events that coincide with taking statins aren't necessarily caused by them
Statin muscle pain for some, but not universally, and now we understand more about why
Dosing isn't one-size-fits-all: Personalized approaches considering individual factors may work better
Risk-benefit calculations differ: For someone with established coronary disease, benefits typically outweigh risks; for primary prevention in low-risk people, this requires more careful consideration
The goal isn't to demonize statins or to suggest they're safe for everyone in all doses. Rather, it's to acknowledge what the evidence actually shows: statins can be powerful tools for preventing cardiovascular disease in appropriate patients, but they work best when prescribed thoughtfully, with attention to individual factors and close monitoring of actual effects versus coincidental events.
Author’s Note
Statins remain one of the most extensively studied and most frequently prescribed medications in modern medicine—yet few drugs generate as much confusion, fear, and debate among patients and clinicians alike. Over the years, I have encountered countless individuals who discontinued statin therapy not because of proven harm, but because of uncertainty, alarming package inserts, or anecdotal experiences amplified far beyond what high-quality evidence supports. At the same time, I have also seen patients whose symptoms were real, distressing, and too easily dismissed.
This article was written to bridge that gap. The goal is neither to defend statins uncritically nor to undermine their proven cardiovascular benefits. Instead, it is to present what the best contemporary evidence actually shows—especially evidence from double-blind randomized controlled trials that allow us to distinguish true drug-related adverse effects from coincidental or nocebo-driven symptoms. Recent advances in mechanistic research have also clarified why a subset of patients genuinely experiences statin-related muscle symptoms, reinforcing the need for empathy, validation, and individualized care rather than blanket assumptions.
Medicine works best when decisions are guided by data, context, and patient-specific risk—not fear, absolutes, or one-size-fits-all dogma. Statins can be life-saving for many, unnecessary for some, and poorly tolerated by a few. Recognizing these distinctions is essential for responsible cardiovascular prevention.
I hope that this piece empowers patients to ask better questions and encourages clinicians to have more nuanced, evidence-based conversations—so that statin therapy, when used, is prescribed thoughtfully, monitored carefully, and aligned with each individual’s biology, risk profile, and values.
Disclaimer: This article is for educational purposes only and should not be considered medical advice. Always consult with your healthcare provider before starting, stopping, or changing any medication. Individual responses to statin therapy vary, and treatment decisions should be personalized based on your unique health circumstances, risk factors, and medical history.
Related Articles
Statin Therapy and Dementia Risk: A Critical Review of Current Evidence | DR T S DIDWAL
Your Body Fat Is an Endocrine Organ—And Its Hormones Shape Your Heart Health | DR T S DIDWAL
hsCRP Explained: What Inflammation Means for Your Heart | DR T S DIDWAL
What’s New in the 2025 Blood Pressure Guidelines? A Complete Scientific Breakdown | DR T S DIDWAL
References
Demasi, M., Redberg, R. F., & Abramson, J. D. (2025). Statins for primary prevention of cardiovascular disease. JAMA, 333(10), 908–909. https://doi.org/10.1001/jama.2024.26588
Dimmitt, S. B., Stampfer, H. G., Kennedy, M. C., & Martin, J. H. (2026). Rethinking statin dosage in coronary disease. Pharmacology Research & Perspectives, 14(1), e70187. https://doi.org/10.1002/prp2.70187
Reith, C., Blackwell, L., Emberson, J. R., Preiss, D., Zannad, F., et al. (2026). Assessment of adverse effects attributed to statin therapy in product labels: A meta-analysis of double-blind randomised controlled trials. The Lancet, Advance online publication. https://doi.org/10.1016/S0140-6736(25)01578-8
University of British Columbia. (2026, January 31). Scientists finally explain statin muscle pain. ScienceDaily. Retrieved from https://www.sciencedaily.com/releases/2026/01/260131084610.htm