Rebuilding Cellular Powerhouses: The New Science of Autophagy, Mitophagy, and Longevity
Explore how mitophagy repairs damaged mitochondria, supports cellular energy, and protects against aging, metabolic disorders, neurodegeneration, and chronic disease
AGING
Dr. T.S. Didwal, M.D.(Internal Medicine)
1/1/202610 min read

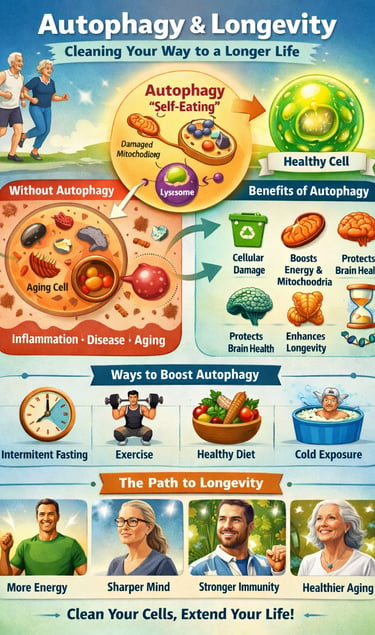
Aging is not merely the passage of time—it is the gradual accumulation of cellular damage. At the center of this process lies autophagy, a highly conserved biological mechanism through which cells identify, degrade, and recycle damaged proteins and organelles. Often described as the cell’s internal “quality-control” system, autophagy is now recognized as a core hallmark of aging and a critical determinant of healthspan and longevity (Carosi et al., 2025).
As autophagy efficiency declines with age, dysfunctional mitochondria, protein aggregates, and metabolic waste accumulate, driving chronic inflammation, mitochondrial dysfunction, immune senescence, and neurodegeneration. Experimental and translational research demonstrates that impaired autophagy is closely linked to age-related disorders, including cardiovascular disease, type 2 diabetes, sarcopenia, and Alzheimer’s disease (Shan et al., 2025).
Importantly, autophagy is not uniform across the body. Recent human and animal studies reveal cell-specific and tissue-specific autophagy signatures, influenced by age, sex, and nutrient availability (Dang & Sargeant, 2025). This insight has reshaped modern longevity science, highlighting why personalized interventions—rather than one-size-fits-all solutions—are essential.
Encouragingly, autophagy can be modulated through evidence-based lifestyle strategies, including intermittent fasting, physical activity, plant-derived bioactive compounds, and sleep optimization (Mundo Rivera et al., 2024). By supporting this intrinsic cellular renewal system, we may not only slow biological aging but also preserve vitality, metabolic health, and cognitive function across the lifespan.
In this comprehensive guide, we'll explore how cellular autophagy, tissue-specific autophagy patterns, and mitochondrial autophagy are reshaping our understanding of aging and offering new hope for age-related diseases.
Clinical pearls
1. The "Tissue Clock" Divergence
Autophagy decline isn't a uniform "on/off" switch across the body. Research on aging tissues shows that the brain and heart experience much steeper drops in recycling capacity than other organs
Don't wait for "full-body" symptoms to start. Because your brain is most sensitive to cellular "trash" buildup, starting autophagy-supporting habits like HIIT or intermittent fasting now is primarily a move for neurological insurance.
2 Sex-Specific Cellular Cleaning
Autophagy signatures in immune cells (leukocytes) differ significantly between biological men and women. This suggests that the "optimal" fasting window or exercise intensity for cellular cleanup may look different depending on your sex.
Use a personalized approach. Women may find better success with more moderate, consistent fasting windows (12–14 hours), while men may require slightly longer windows to reach the same "autophagy threshold."
3. Mitophagy: The Powerhouse Restoration
General "cellular cleaning" is good, but mitophagy—the targeted recycling of damaged mitochondria—is what actually restores energy levels . Accumulating "broken" mitochondria is a primary driver of chronic fatigue and inflammation in aging.
Think of mitophagy as "swapping out old batteries." To trigger this specific cleanup, focus on zone 2 aerobic exercise and cold exposure, which are the most potent natural triggers for mitochondrial turnover.
4. Natural "Cleaning Signals" (Polyphenols)
You don't need pharmaceutical "autophagy mimetics" to activate your cleanup crews. Natural compounds like EGCG (Green Tea), Resveratrol (Berries), and Curcumin act as chemical signals that flip the autophagy switch
Treat your spice cabinet and produce drawer like a pharmacy. Incorporating deep pigments (blueberries, turmeric, dark leafy greens) provides the biochemical "key" that unlocks your cells' built-in maintenance systems.
5.The "Hormetic Stress" Sweet Spot
Autophagy is a response to hormetic stress—short, controlled bursts of "hardship" that don't kill the cell but force it to get more efficient.
Avoid "chronic comfort." Your cellular maintenance crew only shows up when there is a perceived need. Brief periods of hunger (fasting), temperature changes (cold showers), or physical exertion (HIIT) are the only ways to "call the crew" to duty.
Autophagy and Aging: How Your Cells' Cleaning System Holds the Key to Longevity
Autophagy is your body's cellular recycling system. At the molecular level, this process involves encapsulating damaged proteins, organelles, and other cellular waste in a membrane-bound compartment called an autophagosome. These autophagosomes then fuse with lysosomes—cellular structures containing digestive enzymes—that break down the contents into reusable building blocks.
Think of it as your cells' spring cleaning on a microscopic level. When functioning optimally, autophagy removes:
Damaged proteins that can aggregate and cause disease
Worn-out mitochondria (the cellular powerhouses)
Accumulated cellular debris
Pathogens and foreign invaders
Excess fat stores
Without this cleanup mechanism, cells accumulate toxic substances that contribute to inflammation, neurodegeneration, and premature aging. This is why autophagy activation has become such a hot topic in longevity research.
Autophagy vs. Mitophagy: General Cleanup vs. Powerhouse Precision
While often used interchangeably, these two processes represent different "tiers" of cellular maintenance. Understanding the distinction is vital for a targeted longevity strategy.
Autophagy (The Generalist): This is your cell’s general recycling program. It acts like a city-wide garbage service, patrolling the cell to collect and recycle misfolded proteins, accumulated metabolic waste, and damaged membranes. Its primary role is to prevent the "clutter" that leads to systemic inflammation and neurodegeneration.
Mitophagy (The Specialist): This is a highly selective form of autophagy that targets only the mitochondria. When your cellular "power plants" become damaged, they stop producing clean energy and start leaking toxic oxidative stress. Mitophagy acts as a precision demolition crew, identifying and removing these failing batteries before they can damage the cell.
Why it matters: While general autophagy keeps the cellular environment clean, mitophagy is what determines your metabolic age. By replacing old, "leaky" mitochondria with new, efficient ones, you directly improve energy levels and reduce the oxidative damage associated with aging.
Clinical Tip: While fasting is the primary driver of general autophagy, Zone 2 exercise and cold exposure are the most potent triggers for mitophagy.
Cell Type-Specific Autophagy: Understanding Individual Cellular Responses
Study 1: Cell Type-Specific Autophagy in Human Leukocytes
One of the most intriguing discoveries in recent autophagy research comes from examining how different immune cells respond to aging and metabolic stress. Dang and Sargeant' et al.( 2025) in Autophagy Reports reveals that autophagy doesn't work the same way in every cell type.
Leukocytes—the white blood cells that defend your body against infection—show distinct autophagy signatures that change with aging, biological sex, and nutritional status. This means that a person's age, whether they're male or female, and their current diet all influence how efficiently their immune cells can clean house.
Key Takeaways:
Different leukocyte populations have unique autophagy patterns
Aging diminishes autophagy capacity in immune cells, potentially explaining age-related immune dysfunction
Sex-based differences in autophagy suggest that anti-aging interventions may need to be personalized for men and women
Nutrient restriction triggers distinct autophagy responses in immune cells, linking intermittent fasting and caloric restriction to immune health
Understanding cell-type-specific autophagy could lead to targeted therapies that enhance immune function in aging populations
This research emphasizes that autophagy is not a one-size-fits-all process. To develop truly effective anti-aging strategies, we must consider how individual cell types respond differently to the same stimuli.
Autophagy Across Tissues: A Comprehensive Look at Aging
Study 2: Autophagy Across Tissues of Aging Mice
While understanding individual cell types is important, aging affects the entire organism. The comprehensive study by Carosi et al (2025) examined tissue-specific autophagy patterns across multiple organs in aging mice. This research provides a systems-level perspective on how autophagy dysfunction contributes to aging.
The study systematically compared autophagy activity in young versus old mice across various tissues—including the brain, heart, liver, skeletal muscle, and immune organs. The results paint a sobering picture: across virtually every tissue examined, autophagy capacity declines with age.
Key Takeaways:
Age-dependent autophagy decline occurs across multiple organ systems
Different tissues show varying rates of autophagy deterioration—some tissues become autophagy-deficient earlier than others
The universal decline in tissue autophagy correlates with the development of age-related pathology
Brain tissues and cardiac tissues show particularly steep autophagy decline, which may explain high rates of neurodegenerative and cardiovascular diseases in aging
Preserving autophagy capacity in critical tissues could be a universal strategy for healthy aging
This research supports the concept that aging itself is fundamentally a disease of impaired cellular recycling
This multi-tissue analysis suggests that any effective anti-aging therapy must address autophagy dysfunction at a systemic level, not just in isolated cell types.
Mitochondrial Autophagy: Targeting the Powerhouse
Study 3: Targeting Mitochondrial Autophagy for Anti-Aging
Citation: Shan, W., Liu, Y., Tang, R., et al. (2025). Targeting mitochondrial autophagy for anti-aging. Cell Death Discovery, 4.
While general autophagy is important, emerging research suggests that mitochondrial autophagy (also called mitophagy) may be even more critical for healthy aging. Mitochondria are the energy factories of your cells, but they're also major producers of harmful reactive oxygen species (ROS) when they malfunction.
Shan et al.'s (2025 )research in Cell Death Discovery focuses on this specialized form of autophagy, where cells specifically target and recycle damaged mitochondria. This is particularly important because dysfunctional mitochondria accumulate with age and are major drivers of age-related diseases.
Key Takeaways:
Mitochondrial autophagy is a selective, targeted process distinct from general autophagy
Aging impairs the ability to recognize and eliminate damaged mitochondria
Accumulation of dysfunctional mitochondria drives mitochondrial dysfunction, leading to increased oxidative stress
Targeting mitophagy with specific interventions could prevent the mitochondrial decline that characterizes aging
Enhancing mitochondrial autophagy may be particularly important for tissues with high energy demands (brain, heart, muscle)
This approach represents a shift from general anti-aging strategies to more specific, organelle-targeted interventions
Research supports the concept that mitochondrial health is foundational to cellular health and longevity
The implications are significant: by selectively targeting mitochondrial cleanup, we might be able to maintain cellular energy production and reduce oxidative damage—two hallmarks of healthy aging.
Natural Autophagy Activators: From Lab to Life
Study 4: Natural Autophagy Activators to Fight Age-Related Diseases
While understanding the science is crucial, the practical question becomes: how do we enhance autophagy? Mundo Rivera et al.( 2024) review in Cells explores natural autophagy activators—compounds found in food and plants that can turn on the body's cleanup system.
This research transitions from basic science to applied nutrition, examining how dietary and herbal components can trigger autophagy without pharmaceutical intervention.
Key Takeaways:
Numerous natural compounds can activate autophagy, including polyphenols, terpenoids, and alkaloids
Foods like green tea, berries, and cruciferous vegetables contain potent autophagy activators
Plant-based compounds offer accessible ways to enhance cellular recycling without medication
Natural activators may work synergistically with other anti-aging interventions
Understanding the molecular mechanisms of these natural compounds can help develop more effective therapeutic strategies
This research bridges the gap between traditional medicine and modern molecular biology
Incorporating autophagy-activating foods into your diet may be a practical first step in supporting cellular health
This study is particularly exciting because it suggests that you don't need expensive drugs or high-tech interventions—nature has already provided many tools for enhancing autophagy.
The Aging Connection: Why Autophagy Matters for Longevity
Aging is characterized by progressive functional decline across all body systems. Recent research reveals that a central culprit in this decline is impaired autophagy. Here's why this matters:
Cellular Debris Accumulation: Without effective autophagy, damaged proteins, lipids, and organelles accumulate inside cells, triggering inflammation and cellular dysfunction.
Mitochondrial Decline: As mitochondria age and autophagy fails to remove them, energy production plummets while oxidative stress rises. This creates a vicious cycle of cellular damage.
Immune Dysfunction: As shown in the leukocyte study, impaired autophagy in immune cells compromises our ability to fight infections and clear out aberrant cells.
Neurodegeneration: The brain is particularly vulnerable to autophagy defects. Protein aggregates characteristic of Alzheimer's, Parkinson's, and other neurodegenerative diseases accumulate when autophagy fails.
Metabolic Dysregulation: Impaired autophagy disrupts energy homeostasis, contributing to obesity, insulin resistance, and metabolic syndrome.
Practical Applications: How to Enhance Your Autophagy
Based on the research covered here, several evidence-based strategies can support your body's autophagy:
Intermittent Fasting and Caloric Restriction
Both trigger autophagy as cells must recycle internal components for energy. Research shows benefits starting with 12-16 hour fasting windows, though individual tolerance varies.
Nutrient-Dense, Plant-Based Eating
Foods rich in polyphenols and other autophagy activators include:
Green and white tea
Berries (especially blueberries and cranberries)
Cruciferous vegetables (broccoli, cauliflower, Brussels sprouts)
Dark chocolate (70% cacao or higher)
Turmeric and other spices
Regular Physical Activity
Exercise is a powerful autophagy trigger, particularly resistance training and high-intensity interval training (HIIT).
Adequate Sleep
During deep sleep, autophagy naturally increases. Poor sleep quality impairs this process, accelerating aging.
Stress Management
Chronic stress suppresses autophagy. Meditation, yoga, and other stress-reduction techniques can help maintain autophagy capacity.
Cold Exposure
Brief cold exposure (cold showers, ice baths) has been shown to activate autophagy, though this should be done cautiously.
FAQ: Autophagy and Aging
Q: Can autophagy really slow aging? A: While autophagy enhancement shows tremendous promise in research models, the evidence in humans remains preliminary. However, the mechanisms strongly suggest that maintaining healthy autophagy is fundamental to healthy aging.
Q: How long does autophagy take to activate? A: Autophagy begins to increase within 4-8 hours of fasting or exercise, with more pronounced effects after 12-16 hours.
Q: Are autophagy supplements effective? A: Most over-the-counter autophagy supplements have limited scientific support. Natural dietary approaches with established mechanisms are generally preferable.
Q: Can you have too much autophagy? A: Yes—excessive autophagy (particularly if induced artificially) can damage healthy cells. Balance is key, which natural approaches tend to support better than forced interventions.
Q: Is autophagy the same for men and women? A: No. The Dang and Sargeant research shows sex-specific differences in autophagy patterns, suggesting personalized approaches may be optimal.
Q: Which organs most benefit from enhanced autophagy? A: The brain, heart, liver, and skeletal muscle show the most dramatic age-related autophagy decline and likely benefit most from interventions.
Q: How quickly can I see benefits from autophagy-enhancing practices? A: Molecular changes occur within hours of fasting or exercise, but measurable health improvements typically take weeks to months of consistent practice.
The Future of Anti-Aging: Beyond Autophagy
While autophagy is undeniably important, it's one piece of a larger puzzle. The most effective anti-aging strategies will likely combine autophagy enhancement with other interventions addressing cellular senescence, telomere shortening, epigenetic aging, and inflammation.
However, given that autophagy dysfunction appears to be a central mechanism driving multiple aging pathways, enhancing this process should be foundational to any serious anti-aging protocol.
Call to Action: Take Control of Your Cellular Health
The research is clear: your cells have an amazing ability to repair and renew themselves, but this capacity declines without support. You don't need to wait for pharmaceutical breakthroughs to take advantage of what we know today.
Start implementing autophagy-supporting practices now:
Establish a regular fasting window (even 12-14 hours overnight)
Increase plant-based whole foods in your diet
Move your body daily, especially with resistance training
Prioritize 7-9 hours of quality sleep
Find ways to manage stress that resonate with you
Track how you feel over the next 30 days. Most people report increased energy, better mental clarity, improved digestion, and enhanced overall well-being when they support their autophagy naturally.
Remember: longevity isn't just about adding years to your life—it's about adding life to your years. By supporting your cells' natural cleanup system, you're investing in the most foundational aspect of health.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Cellular Senescence: Why Your Cells Age and What It Means for Your Health | DR T S DIDWAL
Exercise and Longevity: The Science of Protecting Brain and Heart Health as You Age | DR T S DIDWAL
The Science of Healthy Brain Aging: Microglia, Metabolism & Cognitive Fitness | DR T S DIDWAL
The Aging Muscle Paradox: How Senescent Cells Cause Insulin Resistance and The Strategies to Reverse It | DR T S DIDWAL
VO2 Max & Longevity: The Ultimate Guide to Living Longer | DR T S DIDWAL
Waist-Calf Ratio: The Longevity Metric Most People Aren’t Tracking | DR T S DIDWAL
Blue Zones Secrets: The 4 Pillars of Longevity for a Longer, Healthier Lifepost | DR T S DIDWAL
Anabolic Resistance: Why Muscles Age—and How to Restore Their Growth Response | DR T S DIDWAL
References
Carosi, J. M., Martin, A., Hein, L. K., Hassiotis, S., Hattersley, K. J., Turner, B. J., Fourrier, C., Bensalem, J., & Sargeant, T. J. (2025). Autophagy across tissues of aging mice. PloS ONE, 20(6), e0325505. https://doi.org/10.1371/journal.pone.0325505
Dang, L. V., & Sargeant, T. J. (2025). Cell type-specific autophagy in human leukocytes: signatures of aging, sex, and nutrient restriction. Autophagy Reports, 4(1). https://doi.org/10.1080/27694127.2025.2543560
Mundo Rivera, V. M., Tlacuahuac Juárez, J. R., Murillo Melo, N. M., Leyva Garcia, N., Magaña, J. J., Cordero Martínez, J., & Jiménez Gutierrez, G. E. (2024). Natural autophagy activators to fight age-related diseases. Cells, 13(19), 1611. https://doi.org/10.3390/cells13191611
Shan, W., Liu, Y., Tang, R., et al. (2025). Targeting mitochondrial autophagy for anti-aging. Cell Death Discovery, 4. https://doi.org/10.1038/s41420-025-02913-y