Can Stem Cells Slow Aging? Cutting-Edge Research on Regeneration, Senolytics, and Immune Therapy
Discover how stem cell therapy, senolytics, and immune cell-based interventions may slow aging. Learn the latest science behind regenerative medicine and longevity.
AGING
Dr. T.S. Didwal, M.D.
11/24/202512 min read
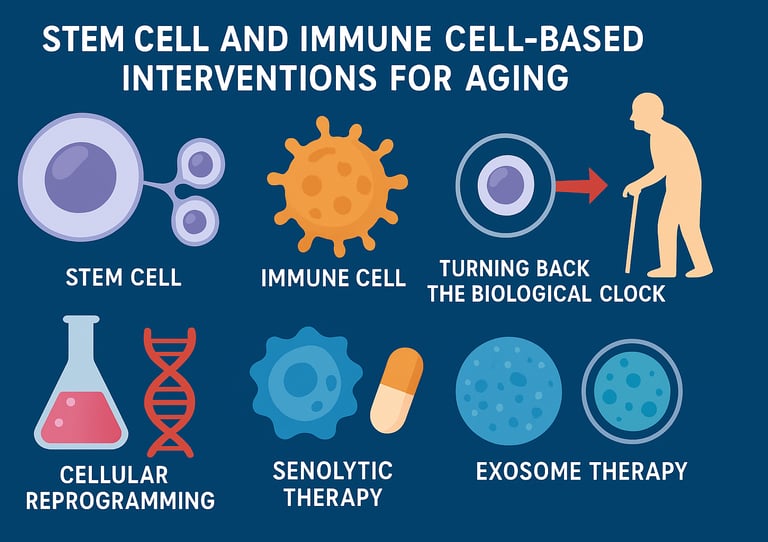

The quest for the fountain of youth has captivated humanity for millennia. While we haven't discovered a magical elixir, modern science is uncovering something potentially more powerful: stem cell-based therapies and immune cell interventions that may slow, halt, or even reverse aspects of aging. These cutting-edge treatments are transitioning from laboratory curiosities to clinical realities, offering hope for extending not just lifespan, but healthspan—the period of life spent in good health
Clinical Pearls
Dual-System Targeting is Key: Effective anti-aging strategies must address both Stem Cell Exhaustion (loss of regenerative capacity) and Immunosenescence (decline in immune function). Combination therapies that address these two systems simultaneously are likely to yield the best results.
Paracrine Signaling vs. Engraftment: Many therapeutic benefits of Mesenchymal Stem Cells (MSCs) come from their paracrine effects (secreting beneficial factors and exosomes), rather than permanent tissue engraftment. This makes Exosome Therapy a promising, cell-free alternative for future standardization and delivery.
Eliminate "Zombie Cells" First: A critical intervention is Senolytic Therapy (e.g., using dasatinib and quercetin) to eliminate senescent cells. This action directly reduces the chronic, damaging inflammation (Inflammaging) caused by the SASP (Senescence-Associated Secretory Phenotype).
Partial Reprogramming is the Balanced Goal: While full cellular reprogramming (to iPSCs) can reset the biological clock, it poses a risk of tumor formation. Safer clinical efforts focus on Partial or Transient Reprogramming to restore youthful gene expression without the high cancer risk of full pluripotency.
Exercise Caution Regarding Clinics: Stem cell treatments remain largely experimental. Always seek treatments delivered through registered clinical trials or major academic medical centers. High costs, guaranteed results, and offering one therapy for many unrelated diseases are significant red flags for unproven treatments.
Understanding the Cellular Basis of Aging
Before diving into interventions, let's explore why we age at the cellular level. Aging isn't simply the passage of time; it's a complex biological process driven by multiple interconnected mechanisms that affect our stem cells and immune system.
The Stem Cell Aging Framework
Recent research has established a comprehensive framework for understanding stem cell aging. According to Liu et al. (2022), stem cell aging involves nine interconnected hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Think of these as nine dominoes—when one falls, it often triggers others, creating a cascade of aging effects throughout your body.
What makes this framework particularly important is that stem cells serve as your body's repair crew. These remarkable cells can self-renew and differentiate into specialized cell types, replacing damaged tissues throughout your life. However, as stem cells age, their regenerative capacity diminishes, contributing to the functional decline we associate with getting older.
The Dual Nature of Stem Cells in Aging
Here's where things get interesting: stem cells play a paradoxical role in aging. On one hand, they're essential for tissue maintenance and repair. On the other, their age-related dysfunction accelerates the aging process itself. Chaudhary et al. (2025) highlight this duality, explaining how age-related diseases like cardiovascular disorders, neurodegenerative conditions, metabolic syndromes, and musculoskeletal deterioration are all linked to stem cell dysfunction.
The study emphasizes that different tissue-specific stem cells—including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), neural stem cells (NSCs), and cardiac stem cells—all undergo age-related changes. These changes manifest as reduced self-renewal capacity, impaired differentiation potential, and accumulation of cellular damage. It's like having a construction crew that becomes less skilled and motivated over time—the building (your body) inevitably starts to show signs of neglect.
Revolutionary Stem Cell Therapies: From Bench to Bedside
The transition from understanding stem cell aging to developing clinical interventions represents one of medicine's most exciting frontiers. Let's explore the various therapeutic approaches currently being investigated and implemented.
Mesenchymal Stem Cells: The Versatile Healers
Mesenchymal stem cells (MSCs) have emerged as particularly promising candidates for anti-aging therapies. El Assaad et al. (2024) conducted a comprehensive scoping review of stem cell-based anti-aging approaches, revealing that MSCs possess unique properties that make them ideal for combating age-related decline.
What makes MSCs special? These cells can be sourced from multiple tissues including bone marrow, adipose tissue, and umbilical cord, and they possess three critical capabilities: they can differentiate into various cell types, secrete beneficial factors (paracrine effects), and modulate immune responses. The review identified MSC applications across diverse areas including facial aesthetics, hair restoration, orthopedic conditions, and systemic anti-aging effects.
One fascinating aspect is that MSCs don't necessarily need to engraft permanently to be effective. Often, their therapeutic benefit comes from the bioactive molecules they secrete—growth factors, cytokines, and exosomes—that signal other cells to repair and regenerate. It's like sending in expert consultants who advise your existing repair crews rather than replacing them entirely.
Pluripotent Stem Cells: Resetting the Aging Clock
Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) represent another powerful approach. He et al. (2025) discuss how these cells can be reprogrammed to an embryonic-like state, essentially resetting their biological age. This cellular reprogramming technology, pioneered by Nobel laureate Shinya Yamanaka, involves introducing specific transcription factors (Oct4, Sox2, Klf4, and c-Myc—collectively known as OSKM factors) into adult cells.
The beauty of iPSC technology is that it offers the potential for personalized medicine. Cells can be taken from a patient, reprogrammed to a youthful state, differentiated into needed cell types, and transplanted back without immune rejection concerns. However, challenges remain, including the risk of tumor formation and the need for precise control over differentiation processes.
Partial Reprogramming: A Balanced Approach
An innovative middle ground between full reprogramming and traditional stem cell therapy is partial reprogramming or transient reprogramming. This approach briefly exposes cells to reprogramming factors without fully converting them to pluripotency. The result? Cells regain some youthful characteristics while maintaining their specialized identity and avoiding the cancer risks associated with fully pluripotent cells.
Immune Cell-Based Interventions: Rejuvenating Your Body's Defense System
While stem cells steal much of the spotlight, immune system aging (immunosenescence) is equally critical to understanding and combating aging. Your immune system doesn't just fight infections—it plays crucial roles in tissue maintenance, cancer surveillance, and coordinating repair processes throughout your body.
Understanding Immunosenescence
Immunosenescence refers to the age-related deterioration of immune function. He et al. (2025) explain how this process involves thymic involution (shrinking of the thymus gland), decreased T cell diversity, accumulation of senescent immune cells, chronic low-grade inflammation (inflammaging), and impaired responses to new pathogens. This explains why older adults are more susceptible to infections, show reduced vaccine responses, and have higher cancer rates.
Senolytic Therapies: Eliminating Zombie Cells
One of the most exciting developments in anti-aging interventions is senolytic therapy—treatments that selectively eliminate senescent cells. These "zombie cells" have stopped dividing but refuse to die, instead secreting inflammatory molecules that damage surrounding tissues through what's called the senescence-associated secretory phenotype (SASP).
Wang et al. (2025) discuss how MSCs can be engineered or combined with senolytic drugs to target and eliminate these problematic cells. Early clinical trials have shown promising results, with senolytic combinations like dasatinib and quercetin demonstrating improvements in physical function, inflammation markers, and age-related pathologies.
CAR-T and Immune Cell Engineering
Chimeric antigen receptor T-cell (CAR-T) therapy, already revolutionary in cancer treatment, is being adapted for aging interventions. He et al. (2025) describe how CAR-T cells can be engineered to recognize and destroy senescent cells, creating a sort of immune-based "search and destroy" mission against aging cells.
Additionally, enhancing the function of natural killer (NK) cells, regulatory T cells (Tregs), and other immune populations offers multiple avenues for intervention. These approaches aim to restore youthful immune surveillance and reduce chronic inflammation.
Thymus Regeneration: Rebuilding Immune Central Command
Since thymic involution is a key driver of immunosenescence, several strategies aim to regenerate or rejuvenate the thymus. These include growth factor administration (like IL-7 and keratinocyte growth factor), sex steroid ablation (since sex hormones accelerate thymic aging), and stem cell-based approaches to rebuild thymic tissue. Regenerating the thymus could restore the body's ability to produce diverse, functional T cells throughout life.
Clinical Applications: Where Theory Meets Practice
The transition from promising laboratory results to effective clinical treatments requires rigorous testing and validation. Let's examine where we currently stand in translating these discoveries into real-world therapies.
Current Clinical Landscape
Wang et al. (2025) provide a qualitative review of MSC clinical applications in treating immunosenescence. Their analysis reveals both exciting potential and significant challenges. MSCs have shown immunomodulatory effects in various clinical contexts, helping to regulate excessive inflammation and support tissue repair.
Current clinical trials are investigating MSC therapies for conditions closely linked to immunosenescence, including frailty syndrome, age-related cognitive decline, osteoarthritis, cardiovascular diseases, and metabolic disorders. Early results suggest improvements in inflammatory markers, physical function, and quality of life measures.
Safety and Efficacy Considerations
Not all results are created equal. El Assaad et al. (2024) found considerable variability in study designs, outcome measures, and reporting standards across the stem cell therapy literature. Many studies are observational or lack proper controls, making it difficult to draw definitive conclusions about efficacy.
Safety concerns include potential for tumor formation (particularly with pluripotent cells), immune reactions, improper differentiation, and transmission of pathogens. Regulatory bodies worldwide are working to establish appropriate standards for regenerative medicine products, but the field remains somewhat like the Wild West—exciting opportunities coexist with legitimate concerns about unproven treatments marketed directly to consumers.
The Route of Administration Matters
How stem cells are delivered significantly impacts their therapeutic effect. Common routes include intravenous infusion (systemic distribution), local injection (targeted delivery), and topical application (for skin-related applications). Wang et al. (2025) note that each approach has distinct advantages and limitations regarding cell survival, engraftment, and therapeutic impact.
Emerging Technologies and Future Directions
The field of regenerative medicine and anti-aging interventions is evolving rapidly. Several cutting-edge approaches are on the horizon that could revolutionize how we approach aging.
Exosome Therapy: Cell-Free Regeneration
Exosomes—tiny vesicles secreted by cells—are gaining attention as a cell-free alternative to direct stem cell transplantation. These nanoscale packages contain proteins, RNAs, and other signaling molecules that can promote tissue repair and modulate immune responses. He et al. (2025) highlight how exosome therapy might offer many benefits of stem cell treatments while avoiding complications related to living cell transplantation.
The advantages are compelling: exosomes are easier to store and standardize, have lower immunogenicity, can cross biological barriers more easily, and eliminate concerns about improper cell differentiation. Think of them as sending instruction manuals rather than the construction crew itself.
Epigenetic Reprogramming
Rather than replacing aged cells, epigenetic interventions aim to rejuvenate them in place. Liu et al. (2022) discuss how age-related epigenetic changes—modifications to DNA and histones that affect gene expression without changing the genetic sequence—can potentially be reversed.
Approaches include small molecules that modulate epigenetic enzymes (like histone deacetylase inhibitors), dietary interventions affecting DNA methylation, and direct epigenetic editing using CRISPR-based tools. This represents a shift from replacing dysfunctional cells to resetting their molecular clocks.
Combination Therapies: A Multi-Pronged Approach
Given aging's complexity, single interventions may prove insufficient. Chaudhary et al. (2025) emphasize that the most effective strategies will likely combine multiple approaches—stem cell therapies with senolytics, immune modulation with metabolic interventions, and cellular treatments with lifestyle modifications.
This makes biological sense: if aging results from nine interconnected hallmarks, targeting just one leaves the others unchecked. Combination therapies could address multiple aging mechanisms simultaneously, producing synergistic effects greater than the sum of individual treatments.
Biomarkers and Personalized Interventions
A major challenge in aging research is measuring success. Unlike treating acute diseases with clear endpoints, combating aging requires tracking subtle, long-term changes across multiple biological systems. The development of robust aging biomarkers—including epigenetic clocks, inflammatory markers, metabolic indicators, and functional assessments—is crucial for personalizing interventions and tracking their effectiveness.
He et al. (2025) discuss how advances in systems biology and artificial intelligence are enabling more sophisticated analysis of individual aging trajectories, potentially allowing tailored intervention strategies based on a person's unique biological profile.
Challenges and Ethical Considerations
Despite tremendous progress, significant hurdles remain before stem cell and immune cell-based anti-aging therapies become mainstream medicine.
Scientific and Technical Challenges
Wang et al. (2025) identify several key challenges in translating MSC therapies to clinical practice: donor variability (MSCs from different donors or even different tissues in the same donor show variable properties), manufacturing standardization (producing consistent, high-quality cell products at scale), optimal dosing and timing (determining how many cells to administer and how frequently), and long-term safety monitoring (tracking potential late-emerging complications).
Liu et al. (2022) add concerns about the tumor-forming potential of some stem cell interventions, particularly those involving pluripotent cells or extensive genetic manipulation. Balancing regenerative potential against cancer risk remains a critical consideration.
Regulatory and Economic Barriers
The regulatory landscape for regenerative medicine is complex and evolving. Different countries have varying standards, creating confusion for developers and patients alike. The high cost of developing and manufacturing cellular therapies raises questions about accessibility—will these interventions only be available to the wealthy, or can they be scaled to benefit broader populations?
Ethical Questions
Profound ethical questions surround longevity interventions: Should we intervene in the aging process at all? How do we define the boundary between treating disease and enhancing normal function? What are the societal implications of dramatically extended lifespans? Who should have access to these technologies? These questions have no easy answers and require ongoing dialogue among scientists, ethicists, policymakers, and the public.
Practical Steps: What Can You Do Now?
While cutting-edge cellular therapies remain largely experimental, you're not powerless against aging. Research consistently shows that lifestyle interventions can profoundly influence how your stem cells and immune system age.
Evidence-Based Lifestyle Approaches
Chaudhary et al. (2025) emphasize that maintaining stem cell health through lifestyle choices is both practical and scientifically supported. Key approaches include regular physical activity (exercise has been shown to maintain stem cell function and reduce inflammation), caloric restriction or intermittent fasting (demonstrated to enhance stem cell self-renewal and longevity pathways), adequate sleep (essential for immune function and cellular repair), stress management (chronic stress accelerates immunosenescence), and avoiding toxins (smoking, excessive alcohol, and environmental pollutants damage stem cells).
Stay Informed and Engaged
The field is moving quickly. Staying informed about legitimate advances while maintaining healthy skepticism about overhyped claims is important. Reputable sources include peer-reviewed journals, academic medical centers conducting clinical trials, and regulatory agencies like the FDA and EMA.
Frequently Asked Questions
Q: Are stem cell anti-aging treatments available now? A: Some stem cell therapies are available through clinical trials or in certain countries with less stringent regulations. However, most anti-aging applications remain experimental. Be cautious of clinics offering unproven treatments outside of properly supervised clinical trials.
Q: How much do stem cell therapies cost? A: Costs vary dramatically depending on the type of therapy, source of cells, and location. Experimental treatments can range from free (if participating in clinical trials) to tens of thousands of dollars for procedures offered at private clinics. As technologies mature and scale, costs should decrease.
Q: Can stem cell therapy reverse aging completely? A: No current therapy can completely reverse aging. While some interventions show promise for addressing specific aspects of aging, the process is complex and involves multiple interacting systems. Realistic expectations involve slowing age-related decline and treating specific age-related conditions rather than achieving complete rejuvenation.
Q: Are these treatments safe? A: Safety depends on the specific treatment, source of cells, and administration method. Therapies using autologous cells (from your own body) generally carry lower risks than those using donor cells. Pluripotent stem cells carry higher risks of tumor formation. Always seek treatments through reputable medical centers conducting approved clinical trials.
Q: How do I know if a stem cell clinic is legitimate? A: Legitimate providers will clearly explain the treatment's experimental nature, participate in registered clinical trials, provide detailed informed consent, have appropriate medical credentials and oversight, and make realistic claims about potential benefits. Red flags include guaranteed results, treatment of numerous unrelated conditions with the same approach, and lack of peer-reviewed publications supporting their methods.
Q: What's the difference between stem cell therapy and exosome therapy? A: Stem cell therapy involves transplanting living cells that can differentiate and secrete beneficial factors. Exosome therapy uses only the tiny vesicles that cells release, containing signaling molecules but no living cells. Exosome therapy may offer some benefits with potentially fewer complications, though research is still emerging.
Q: Can lifestyle changes really affect my stem cells? A: Yes! Research demonstrates that factors like exercise, nutrition, sleep, and stress management influence stem cell function, gene expression, and aging trajectories. While lifestyle interventions may not be as dramatic as cellular therapies, they're accessible now and have strong evidence supporting their benefits.
Conclusion: The Road Ahead
We stand at an extraordinary moment in medical history. The convergence of stem cell biology, immunology, genomics, and regenerative medicine is opening unprecedented possibilities for extending healthy human lifespan. The studies reviewed here—from Liu et al.'s comprehensive aging framework to Wang et al.'s clinical application analysis—paint a picture of a field rapidly transitioning from theoretical promise to practical application.
Yet, as with any revolutionary technology, the path forward requires careful navigation. We must balance enthusiasm for potential benefits against realistic assessment of current limitations. We must ensure that safety and efficacy standards are maintained even as we accelerate development timelines. And we must grapple with profound ethical questions about how dramatically extending human healthspan might reshape society.
For now, the most prudent approach combines evidence-based lifestyle interventions with informed engagement in legitimate clinical trials when appropriate. The future of anti-aging medicine will likely involve personalized combinations of cellular therapies, immune modulation, metabolic interventions, and lifestyle optimization—a comprehensive strategy addressing the multiple dimensions of aging simultaneously.
The fountain of youth may not exist as ancient myths imagined it. But through rigorous science and thoughtful application of regenerative medicine, we're discovering something potentially more valuable: the ability to extend not just the quantity of life, but its quality, allowing more people to live longer, healthier, and more vibrant lives.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with qualified healthcare professionals before making changes to your health regimen or starting new treatments.
Related Articles
VO2 Max & Longevity: The Ultimate Guide to Living Longer | DR T S DIDWAL
How Betaine Can Slow Aging: Scientific Insights into This Longevity Molecule | DR T S DIDWAL
References
Chaudhary, J. K., Danga, A. K., Kumari, A., Bhardwaj, A., & Rath, P. C. (2025). Role of stem cells in ageing and age-related diseases. Mechanisms of Ageing and Development, 225, 112069. https://doi.org/10.1016/j.mad.2025.112069
El Assaad, N., Chebly, A., Salame, R., Achkar, R., Bou Atme, N., Akouch, K., Rafoul, P., Hanna, C., Abou Zeid, S., Ghosn, M., & Khalil, C. (2024). Anti-aging based on stem cell therapy: A scoping review. World Journal of Experimental Medicine, 14(3), 97233. https://doi.org/10.5493/wjem.v14.i3.97233
He, L., Han, D., Zong, F., Zhang, Y., Han, Z., & Xu, Z. (2025). Recent progress in stem cell and immune cell-based interventions for aging and age-related disorders. Frontiers in Aging, 6, 1638168. https://doi.org/10.3389/fragi.2025.1638168
Liu, B., Qu, J., Zhang, W., Izpisua Belmonte, J. C., & Liu, G. H. (2022). A stem cell aging framework, from mechanisms to interventions. Cell Reports, 41(3), 111451. https://doi.org/10.1016/j.celrep.2022.111451
Wang, X., Guo, D., He, C., Xiang, A., Zeng, W., Wang, Y., Yang, Y., Mao, W., Xu, H., Wang, H., Fu, X., & Tan, F. (2025). Clinical application of mesenchymal stem cells in immunosenescence: A qualitative review of their potential and challenges. Stem Cell Research & Therapy, 16, 265. https://doi.org/10.1186/s13287-025-04360-z
Keywords: stem cell therapy, anti-aging interventions, mesenchymal stem cells, immunosenescence, cellular reprogramming, regenerative medicine, senolytic therapy, stem cell aging, immune cell-based therapies, age-related diseases, pluripotent stem cells, longevity interventions, tissue-specific stem cells, exosome therapy, aging biomarkers