The Aging Muscle Paradox: How Senescent Cells Cause Insulin Resistance and The Strategies to Reverse It
Explore the link between muscle aging, insulin resistance, and senescent cells, plus evidence-based strategies to restore metabolic health.
AGING
Dr. T.S. Didwal, M.D.
11/26/202514 min read
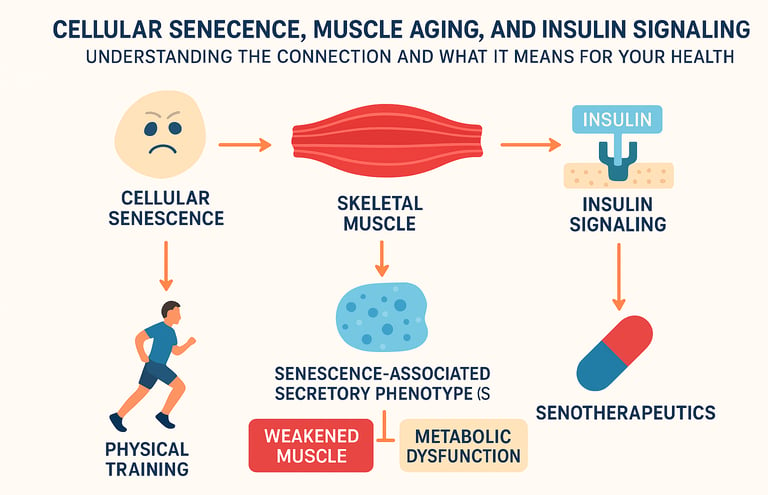
As we age, our bodies undergo profound changes at the cellular level. One of the most fascinating—and concerning—phenomena is cellular senescence, a state where cells stop dividing but refuse to die. When this happens in our skeletal muscles, it triggers a cascade of problems, particularly disrupting how our bodies handle insulin signaling. The result? Weakened muscles, metabolic dysfunction, and a dramatically accelerated aging process. But here's the good news: emerging research is revealing powerful interventions that can reverse this trajectory.
Clinical Pearls
1. Exercise is a First-Line Senolytic and Insulin Sensitizer
The most accessible and powerful intervention for metabolic aging is not pharmacological, but physical. Physical training acts as an endogenous senolytic and senomorphic agent.
Pearl: Don't just prescribe exercise for weight loss or muscle strength (sarcopenia). Emphasize that regular moderate-to-vigorous activity actively reduces the cellular senescence burden in skeletal muscle, thereby addressing the root cause of age-related insulin resistance and metabolic dysfunction (Podraza-Farhanieh et al., 2025).
Actionable Tip: Counsel patients that exercise-induced stress activates natural clearance pathways (autophagy) for senescent cells and promotes mitochondrial health, creating a positive feedback loop that restores insulin sensitivity.
2. Insulin Resistance in Aged Muscle is Driven by Inflammatory "Chatter" (SASP)
The insulin signaling crisis in aged muscle is not primarily due to faulty insulin receptors, but to inflammatory signaling released by senescent cells (the SASP).
Pearl: The inflammatory factors released by senescent muscle cells directly activate p38 Mitogen-Activated Protein Kinase (p38 MAPK). This activation is the critical molecular block that prevents the insulin signaling cascade (PI3K/Akt) from properly translocating the GLUT4 glucose transporter (Rana et al., 2025).
Actionable Tip: The clinical implication is that p38 MAPK inhibitors could serve as a novel class of senomorphics, silencing the damaging SASP without necessarily killing the senescent cell, thereby restoring insulin sensitivity in the muscle microenvironment.
3. Hyperinsulinemia is a Pathological Accelerant of Aging
While insulin is anabolic in youth, the required chronically elevated insulin levels (hyperinsulinemia)—a compensatory response to senescence-driven insulin resistance—may accelerate the aging process itself.
Pearl: The relationship between insulin and aging is paradoxical; chronic hyperinsulinemia is a marker of underlying cellular stress and contributes to the pathological feedback loop that accelerates cellular senescence and age-related disease (Kolb et al., 2023).
Actionable Tip: In older adults with prediabetes/type 2 diabetes, the therapeutic goal should be dual: improve insulin sensitivity by addressing cellular senescence (via exercise or senotherapeutics) to lower required insulin levels, thus breaking the pathological cycle of hyperinsulinemia and accelerated aging.
4. Senotherapeutics Target the Root Cause, Not Just Symptoms
Emerging senolytic and senomorphic drugs represent a shift from managing metabolic symptoms (like high glucose) to addressing the underlying cellular pathology (senescence).
Pearl: Senolytics (drugs that selectively kill senescent cells) and senomorphics (drugs that suppress the SASP) offer the potential for reversal of the aging phenotype in muscle, rather than just slowing decline. The multiomics-driven identification of agents like Maraviroc for sarcopenia highlights this precision approach (Li et al., 2025).
Actionable Tip: Clinicians should stay informed about ongoing clinical trials in senotherapeutics (e.g., Dasatinib, Quercetin, Fisetin, and novel agents) for age-related metabolic dysfunction, as these will likely be incorporated into personalized treatment plans in the coming years.
5. Calcium Dysregulation is a Fundamental Precursor to Muscle Senescence
Beyond SASP and inflammation, dysregulation of calcium signaling is a fundamental, early event in muscle aging that drives both cellular senescence and insulin resistance.
Pearl: Age-related malfunction of calcium-handling proteins (like SERCA and ryanodine receptors) creates a state of calcium dysregulation in the muscle cell. This leads to oxidative stress and mitochondrial dysfunction, which are precursors to cellular senescence accumulation and metabolic chaos (Terrell et al., 2023).
Actionable Tip: Therapeutic strategies targeting the correction of muscle calcium homeostasis (e.g., optimization of Vitamin D, specific calcium-modulating compounds) may represent a complementary, preventive approach to maintain muscle health alongside senolytic and exercise interventions.
Understanding Cellular Senescence: The Cellular Clock That Won't Stop
Imagine a factory worker who shows up to work every day but can't actually perform their job. That's essentially what cellular senescence is. These are cells that have reached the end of their replicative lifespan and have entered a state of permanent growth arrest. They're alive, but they're not truly living in a functional sense.
In skeletal muscle, senescent cells accumulate over time as a natural consequence of aging. What makes this particularly problematic is that these cells don't quietly retire—they actively secrete harmful substances. This phenomenon is called the senescence-associated secretory phenotype (SASP). Think of SASP as your aging cells throwing a tantrum, releasing inflammatory cytokines, growth factors, and other molecular signals that damage surrounding healthy tissue.
The connection between cellular senescence in muscles and insulin resistance has become increasingly clear through contemporary research (Englund et al., 2021). New evidence demonstrates that these senescent muscle cells directly impair insulin signaling pathways, fundamentally altering how your body processes glucose and manages energy metabolism. This isn't just about muscle weakness—it's about systemic metabolic failure.
The Skeletal Muscle Aging Problem: More Than Just Weakness
Skeletal muscle aging represents one of the most clinically relevant aspects of human aging. Muscles aren't just about strength; they're metabolic powerhouses that regulate blood glucose, maintain thermogenesis, and orchestrate hormonal signaling throughout your body. When muscles age, your entire metabolic system suffers.
The conventional understanding of muscle aging focused on sarcopenia—the loss of muscle mass and strength (Englund et al., 2021). But contemporary research reveals that sarcopenia is just the tip of the iceberg. The real culprit is the accumulation of senescent cells within muscle tissue, transforming the muscle microenvironment from anabolic to catabolic.
What makes this even more complicated is that muscle aging isn't a simple linear process. Certain individuals maintain robust, youthful muscles well into their 80s and 90s, while others experience dramatic decline by their 60s. The difference? Insulin sensitivity and the degree of cellular senescence in their tissues. People who maintain healthy insulin signaling appear to be naturally resistant to the accumulation of senescent cells—or possess better mechanisms for clearing them.
The Insulin Signaling Crisis: How Aged Muscles Lose Their Metabolic Control
Insulin signaling is the master switch for glucose uptake, amino acid transport, and protein synthesis in muscle cells. When insulin signaling functions properly, your muscles act as glucose sinks, efficiently clearing blood sugar after meals and maintaining metabolic homeostasis. But when cellular senescence interferes with this pathway, metabolic chaos ensues.
The mechanism involves senescent cells secreting factors that activate inflammatory cascades, particularly through p38 mitogen-activated protein kinase (p38 MAPK) signaling. According to recent research, this activation disrupts the insulin receptor signaling cascade, specifically impairing phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) activation (Rana et al., 2025). When Akt can't be properly activated, glucose transporter 4 (GLUT4) fails to translocate to the cell membrane, and glucose remains in the bloodstream.
The result is insulin resistance in aged muscle, a condition where even normal levels of circulating insulin fail to drive glucose uptake. This creates a vicious cycle: elevated blood glucose triggers compensatory hyperinsulinemia, which accelerates cellular senescence and further impairs insulin signaling. Over time, this progresses to prediabetes and ultimately type 2 diabetes.
Research has identified that calcium dysregulation plays a significant role in this process. Calcium signaling in aging muscle becomes increasingly dysregulated, exacerbating both cellular senescence and insulin resistance (Terrell et al., 2023). This opens new therapeutic targets beyond traditional approaches.
Breakthrough Research: What Recent Studies Reveal
Physical Training as a Cellular Senescence Fighter
One of the most compelling recent findings demonstrates that physical training reduces cellular senescence in skeletal muscle (Podraza-Farhanieh et al., 2025). This isn't just about exercise building muscle—it's about exercise actively clearing senescent cells and restoring insulin signaling.
The mechanism appears to involve multiple pathways. Exercise induces cellular stress responses that activate autophagy and senolytic pathways, essentially triggering the clearance of damaged senescent cells. Simultaneously, exercise promotes mitochondrial biogenesis and improves mitochondrial function, which is fundamental for maintaining healthy, young muscle metabolism. When mitochondria function optimally, insulin signaling improves, and the accumulation of senescent cells decreases. This creates a positive feedback loop where exercise simultaneously clears old cells and promotes new cell adaptation.
Research indicates that physical training simultaneously reduces cellular senescence burden while restoring associated insulin resistance, representing one of the most powerful interventions available for reversing the aging phenotype in muscle (Podraza-Farhanieh et al., 2025).
Key takeaway: Regular physical training is arguably the most powerful intervention for combating cellular senescence in muscle and restoring youthful insulin sensitivity.
Multiomics Reveals the Senescent Muscle Transcriptome
A comprehensive multiomics approach examining aging skeletal muscle identified Maraviroc—a drug originally developed for HIV treatment—as a potential senotherapeutic agent for sarcopenia (Li et al., 2025). This discovery emerged from detailed analysis of gene expression, proteomics, and metabolomic changes in aged muscle tissue.
What makes this finding particularly exciting is the identification of specific molecular signatures that define senescent muscle cells in aging humans. The researchers identified upregulation of senescence markers like p16 and p21, along with dysregulated metabolic pathways (Li et al., 2025). By screening against the senescent transcriptome, they identified compounds like Maraviroc that could selectively target these cells while sparing healthy tissue.
The implications are profound: this suggests we're moving toward a future where senotherapeutics—drugs that selectively eliminate senescent cells—could reverse age-related muscle dysfunction and restore metabolic health (Li et al., 2025). This is distinct from simply treating symptoms; senotherapeutics address the root cause of muscle aging.
Key takeaway: Senotherapeutic drugs targeting cellular senescence in muscle represent a promising frontier for treating sarcopenia and metabolic dysfunction without building new muscle mass.
The SASP-Insulin Resistance Connection
Detailed investigation of the senescence-associated secretory phenotype in cultured muscle cells revealed that senescent cells directly impair glucose homeostasis (Rana et al., 2025). When muscle cells were induced into senescence, they exhibited profound insulin resistance, characterized by reduced GLUT4 translocation and impaired Akt phosphorylation.
Critically, researchers identified that p38 MAPK inhibition could partially reverse the insulin resistance induced by the senescent cell secretome (Rana et al., 2025). This suggests that the inflammatory signaling emanating from senescent cells is mechanistically responsible for impairing insulin signaling. By blocking this communication, insulin sensitivity could be restored.
This finding is transformative because it identifies p38 inhibition as a potential therapeutic strategy. Rather than trying to improve insulin signaling directly through insulin sensitizers, p38 inhibitors could work by silencing the inflammatory chatter of senescent cells, thereby allowing normal insulin signaling to resume.
Key takeaway: P38 MAPK is a critical convergence point between senescence-associated inflammation and insulin resistance, making it an attractive therapeutic target for restoring metabolic function.
Clinical Translation: From Bench to Bedside
The recognition that cellular senescence contributes fundamentally to both insulin resistance and muscle aging has opened new avenues for prevention and treatment (Chaib et al., 2025). Clinical translation efforts are transitioning from focusing solely on glucose control to addressing the underlying cellular dysfunction.
Current clinical approaches increasingly incorporate senolytic and senomorphic strategies into metabolic disease management (Chaib et al., 2025). Senolytics eliminate senescent cells outright, while senomorphics suppress the SASP without killing the cells. This multimodal approach recognizes that different patients may benefit from different interventions based on their individual senescence burden and metabolic phenotype.
For practitioners and patients, this means the future of metabolic disease treatment will likely involve personalized assessment of senescence burden, combined with targeted interventions—whether through exercise, pharmacological senolytics, senomorphics, p38 inhibitors, or some combination thereof.
Key takeaway: Clinical practice is evolving to recognize and address cellular senescence as a primary driver of metabolic disease, with personalized senescence assessment becoming increasingly important.
Calcium Dysregulation: An Underappreciated Link
Calcium signaling in aging muscle has emerged as a critical missing link in the senescence-insulin resistance story (Terrell et al., 2023). Calcium is essential for muscle contraction, but it also plays crucial roles in gene expression, mitochondrial function, and redox homeostasis. With aging, calcium handling becomes dysregulated, contributing to both cellular senescence and insulin resistance.
The mechanism involves age-related changes in calcium-handling proteins like SERCA (sarco/endoplasmic reticulum calcium ATPase) and ryanodine receptors (Terrell et al., 2023). When these proteins malfunction, intracellular calcium becomes dysregulated, triggering oxidative stress and mitochondrial dysfunction. This creates the perfect storm for cellular senescence accumulation and metabolic dysfunction.
Importantly, targeting calcium dysregulation represents a complementary approach to other interventions. While exercise and senolytics address senescent cells directly, managing calcium signaling could prevent senescent cells from forming in the first place (Terrell et al., 2023). This suggests a multi-level intervention strategy where calcium homeostasis serves as a fundamental preventive mechanism.
Key takeaway: Restoring proper calcium signaling in aging muscle may be fundamental for preventing cellular senescence and maintaining metabolic health alongside other interventions.
The Disappointing Relationship Between Insulin and Aging
A critical perspective from aging research suggests that while insulin is essential for young, healthy metabolism, aging fundamentally alters the relationship between organisms and insulin (Kolb et al., 2023). The paradox is striking: in young people, insulin is the ultimate anabolic hormone, driving growth and protein synthesis. But in aging organisms, high insulin levels appear to accelerate aging processes and promote age-related disease.
This paradox is largely explained by cellular senescence. As senescent cells accumulate, the body requires higher insulin levels to maintain glucose homeostasis—a condition known as hyperinsulinemia (Kolb et al., 2023). The chronically elevated insulin further accelerates aging, creating a pathological feedback loop. This highlights why addressing the root cause—cellular senescence itself—is more fundamental than simply trying to improve insulin sensitivity through conventional means.
Research indicates this represents one of the most disappointing findings in aging research: that insulin, essential for youth, becomes problematic with age precisely because of the underlying cellular changes driving senescence (Kolb et al., 2023).
Key takeaway: The relationship between insulin and aging is paradoxical; aging disrupts insulin signaling through cellular senescence, creating a situation where the body becomes more dependent on insulin while becoming less responsive to it.
The Integrated Picture: Connecting Senescence, Muscle Aging, and Insulin Dysfunction
When we synthesize all this research, a coherent picture emerges. Cellular senescence in skeletal muscle is the central driver connecting muscle aging and insulin resistance (Englund et al., 2021). This isn't a coincidence of aging—it's a mechanistic relationship where senescent cells directly impair insulin signaling through inflammatory pathways like p38 MAPK (Rana et al., 2025).
This unified model has profound implications. It explains why exercise is so powerful—it clears senescent cells while simultaneously improving mitochondrial function and calcium handling (Podraza-Farhanieh et al., 2025). It explains why emerging senotherapeutics show promise—by eliminating the cells driving metabolic dysfunction, we address the root cause (Li et al., 2025). It explains why targeting single pathways with conventional insulin sensitizers often produces disappointing results—we're treating symptoms while ignoring the pathological cellular state driving them.
The future of aging and metabolic disease management will likely involve multimodal approaches: lifestyle interventions (particularly exercise), senotherapeutic drugs, calcium-modulating compounds, and anti-inflammatory p38 inhibitors, all coordinated based on individual assessments of senescence burden and metabolic dysfunction (Chaib et al., 2025).
Frequently Asked Questions
Q: Can cellular senescence be reversed? A: Senescent cells themselves cannot be "reversed" back to normal function. However, senescent cells can be cleared through senolytics or senomorphic strategies, and the inflammatory signals they produce can be blocked through p38 inhibitors (Rana et al., 2025). Exercise appears to activate natural clearance mechanisms (Podraza-Farhanieh et al., 2025). The goal is elimination or silencing, not reversal.
Q: How much exercise is needed to reduce cellular senescence? A: Research indicates that regular moderate-to-vigorous exercise appears effective at reducing cellular senescence and improving insulin signaling (Podraza-Farhanieh et al., 2025). However, the specific duration and intensity thresholds require individualized assessment based on current fitness level and metabolic status.
Q: Are there supplements or drugs currently available that target cellular senescence? A: Several compounds are in clinical trials, including senolytic drugs and p38 inhibitors (Rana et al., 2025; Chaib et al., 2025). Senotherapeutic approaches like those targeting cellular senescence represent an emerging therapeutic class (Li et al., 2025). Always consult healthcare providers before starting new supplements or medications.
Q: Can cellular senescence be detected? A: Currently, senescence assessment requires tissue biopsy or specialized blood tests measuring circulating senescent cells or senescence markers (Li et al., 2025). Simple clinical tests aren't yet widely available, though this is an active area of research development through multiomics approaches (Li et al., 2025).
Q: What's the relationship between type 2 diabetes and cellular senescence? A: Cellular senescence contributes to insulin resistance, a key factor in type 2 diabetes development (Rana et al., 2025). By reducing senescent cell burden, many of the pathological processes driving type 2 diabetes may be reversed or prevented through targeted interventions (Chaib et al., 2025).
Q: Is calcium supplementation helpful for aging muscle? A: Calcium balance is important, but supplementation alone isn't sufficient to address calcium signaling dysregulation in aging (Terrell et al., 2023). Exercise, vitamin D status, and overall metabolic health are equally or more important for maintaining proper calcium handling in muscle alongside nutritional adequacy.
Q: How do senescent cells impair insulin signaling? A: Senescent cells release inflammatory factors that activate p38 MAPK signaling, which disrupts the insulin receptor cascade and prevents proper GLUT4 translocation (Rana et al., 2025). This mechanism directly explains the insulin resistance observed in aged muscle tissue.
Q: What are senotherapeutics, and how do they differ from traditional treatments? A: Senotherapeutics are drugs that selectively target senescent cells, either by eliminating them (senolytics) or suppressing their inflammatory secretions (senomorphics) (Li et al., 2025; Chaib et al., 2025). Unlike traditional insulin sensitizers that address symptoms, senotherapeutics target the root cellular cause of metabolic dysfunction.
Q: Can the aging muscle phenotype be completely reversed? A: While complete reversal to youthful status may not be achievable in all cases, emerging evidence suggests that addressing cellular senescence, restoring insulin signaling, and correcting calcium dysregulation can dramatically improve muscle function and metabolic health (Podraza-Farhanieh et al., 2025; Chaib et al., 2025).
Key Takeaways
.Cellular senescence in skeletal muscle is a primary driver of both age-related muscle weakness and insulin resistance, representing a unified mechanism explaining how aging disrupts metabolic health (Englund et al., 2021; Podraza-Farhanieh et al., 2025).
• Physical training actively reduces cellular senescence burden while simultaneously restoring insulin signaling, making exercise the most evidence-based intervention currently available (Podraza-Farhanieh et al., 2025).
• Emerging senotherapeutic drugs like Maraviroc represent a novel class of interventions that directly target the root cause of muscle aging by selectively eliminating senescent cells (Li et al., 2025).
• The senescence-associated secretory phenotype directly impairs insulin signaling through p38 MAPK activation, suggesting that p38 inhibitors could restore metabolic function by silencing inflammatory signals from senescent cells (Rana et al., 2025).
• Calcium dysregulation in aging muscle is an underappreciated contributor to both cellular senescence and insulin resistance, representing a complementary therapeutic target (Terrell et al., 2023).
• Clinical practice is transitioning from symptom management toward addressing the underlying cellular senescence burden, with personalized assessment and multimodal interventions becoming increasingly important (Chaib et al., 2025).
• The relationship between aging and insulin is paradoxical—chronic hyperinsulinemia accelerates aging, but addressing cellular senescence may break this cycle and restore healthy insulin sensitivity (Kolb et al., 2023; Chaib et al., 2025).
Call to Action
The science of cellular senescence, muscle aging, and insulin signaling is rapidly advancing, but the gap between laboratory discoveries and clinical implementation remains wide. Here's what you can do:
For individuals concerned about aging and metabolic health: Start incorporating regular physical activity into your routine—evidence strongly supports exercise as the most accessible and powerful intervention for reducing cellular senescence and improving insulin sensitivity (Podraza-Farhanieh et al., 2025). Prioritize both resistance training (to maintain muscle mass) and aerobic activity (to enhance metabolic function). Additionally, discuss with your healthcare provider whether you might be a candidate for clinical trials involving senotherapeutic agents or whether calcium and vitamin D status should be optimized.
For healthcare providers: Consider incorporating assessment of senescence burden into metabolic disease evaluation, particularly for patients with prediabetes or type 2 diabetes (Chaib et al., 2025). Develop personalized intervention plans that address cellular senescence through combined lifestyle and, where appropriate, pharmacological approaches. Stay informed about emerging senotherapeutic options and clinical trial availability (Li et al., 2025; Rana et al., 2025).
For researchers and biotech companies: Continue advancing senotherapeutic development and work toward accessible clinical diagnostics for assessing senescence burden (Li et al., 2025). Bridge the gap between basic science discoveries and clinical implementation by designing pragmatic trials that assess real-world impact on aging and metabolic disease (Chaib et al., 2025).
The convergence of research demonstrating that cellular senescence is the mechanistic link between muscle aging and insulin resistance offers unprecedented opportunity to fundamentally transform how we approach aging and metabolic disease. The interventions are available, the science is compelling, and the time to act is now.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
VO2 Max & Longevity: The Ultimate Guide to Living Longer | DR T S DIDWAL
Why Exercise Is the Most Powerful Anti-Aging Therapy: A Research-Driven Guide | DR T S DIDWAL
References
Chaib, S., Palmer, A. K., Wyles, S. P., et al. (2025). Translating cellular senescence research into clinical practice for metabolic disease. Nature Reviews Endocrinology. https://doi.org/10.1038/s41574-025-01187-9
Englund, D. A., Zhang, X., Aversa, Z., & LeBrasseur, N. K. (2021). Skeletal muscle aging, cellular senescence, and senotherapeutics: Current knowledge and future directions. Mechanisms of Ageing and Development, 200, 111595. https://doi.org/10.1016/j.mad.2021.111595
Kolb, H., Kempf, K., & Martin, S. (2023). Insulin and aging—a disappointing relationship. Frontiers in Endocrinology, 14, 1261298. https://doi.org/10.3389/fendo.2023.1261298
Li, Y., Li, C., Zhou, Q., et al. (2025). Multiomics and cellular senescence profiling of aging human skeletal muscle uncovers Maraviroc as a senotherapeutic approach for sarcopenia. Nature Communications, 16, 6207. https://doi.org/10.1038/s41467-025-61403-y
Podraza-Farhanieh, A., Spinelli, R., Zatterale, F., Nerstedt, A., Gogg, S., Blüher, M., & Smith, U. (2025). Physical training reduces cell senescence and associated insulin resistance in skeletal muscle. Molecular Metabolism, 95, 102130. https://doi.org/10.1016/j.molmet.2025.102130
Rana, K. S., Marwah, M. K., Raja, F. N. S., Dias, I., Hindalekar, Y. S., Al Tahan, M. A., & Bellary, S. (2025). The influence of senescent associated secretory phenotype on glucose homeostasis in C2C12 muscle cells: Insights into potential p38 inhibitor interventions. Journal of Receptors and Signal Transduction, 45(2), 118–127. https://doi.org/10.1080/10799893.2025.2475441
Terrell, K., Choi, S., & Choi, S. (2023). Calcium's role and signaling in aging muscle, cellular senescence, and mineral interactions. International Journal of Molecular Sciences, 24(23), 17034. https://doi.org/10.3390/ijms242317034