Obesity as a Cardiometabolic Disease: New Science & Treatments
Obesity is now recognized as a cardiometabolic disease. Learn how modern therapies reduce diabetes, heart, and kidney risks.
OBESITYMETABOLISM
Dr. T.S. Didwal, M.D.(Internal Medicine)
1/27/202611 min read

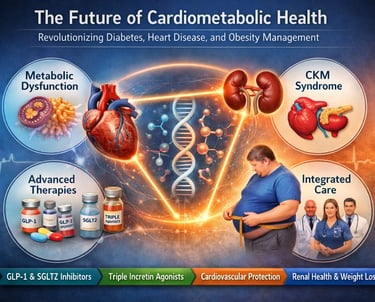
Cardiometabolic disease has quietly become the defining health challenge of the 21st century. Type 2 diabetes, obesity, cardiovascular disease, and chronic kidney disease—once treated as separate clinical entities—are now recognized as interconnected manifestations of a single, progressive pathophysiological continuum. This realization has triggered a profound shift in modern medicine, giving rise to the cardiovascular–kidney–metabolic (CKM) syndrome framework and redefining how clinicians approach prevention, risk stratification, and long-term disease management (Fernando et al., 2025; Pohlman et al., 2025).
At the center of this transformation lies a deeper understanding of metabolic dysfunction—driven by insulin resistance, adipose tissue inflammation, mitochondrial impairment, and neurohormonal dysregulation—as the common upstream driver of cardiometabolic disease progression. Obesity, long misclassified as a lifestyle issue, is now firmly established as a chronic, relapsing cardiometabolic disease that accelerates vascular damage, renal decline, and atherosclerotic risk (Clemmensen et al., 2025).
Simultaneously, therapeutic innovation has entered an unprecedented era. GLP-1 receptor agonists, SGLT2 inhibitors, and emerging dual and triple incretin agonists are no longer viewed solely as glucose-lowering agents. Instead, they are increasingly recognized as disease-modifying cardiometabolic therapies capable of delivering simultaneous benefits across glycemic control, weight reduction, blood pressure regulation, cardiovascular event prevention, and renal protection (Kim, 2025; Pohlman et al., 2025).
Together, these scientific advances signal a paradigm shift—from reactive, organ-specific treatment to integrated, prevention-focused cardiometabolic care. Understanding this transformation is no longer optional; it is essential for clinicians, researchers, and patients navigating the future of metabolic health.
Clinical pearls
1. The "CKM" Mindset: Your Organs are a Network
Modern medicine no longer views the heart, kidneys, and metabolism as separate departments. Think of them as a highly interconnected electrical grid; a surge (dysfunction) in one area, like high blood sugar, inevitably puts strain on the others.
The Pearl: When you manage your weight or blood sugar, you aren't just "treating diabetes"—you are actively shielding your heart and kidneys from future damage.
2. Beyond the Scale: The "Metabolic Quality" of Weight Loss
Newer medications like GLP-1 and Triple Agonists (GLP-1/GIP/Glucagon) are revolutionary not just because they reduce weight, but because they improve how your body handles energy. They reduce "visceral fat" (the dangerous fat around your organs) and quiet systemic inflammation.
The Pearl: Focus on "Metabolic Health" rather than just the number on the scale. Improved energy levels, better blood pressure, and clearer skin are often early signs that your internal metabolic environment is stabilizing.
3. SGLT2 Inhibitors: The "Kidney Vacuum"
Originally for diabetes, SGLT2 inhibitors work by allowing the kidneys to excrete excess glucose through urine. However, the real "pearl" is their secondary effect: they reduce the pressure inside the filters of the kidney and take the "fluid load" off the heart.
The Pearl: Even if your blood sugar is normal, your doctor might suggest these for heart or kidney protection. They act like a relief valve for your circulatory system.
4. The "Legacy Effect" of Early Intervention
Clinical data shows that "metabolic memory" is real. Effectively managing your glucose and blood pressure early in your diagnosis provides protection that lasts for decades, even if management becomes more difficult later in life.
The Pearl: Treat your health like a retirement fund. The "compound interest" of good metabolic control in your 40s and 50s pays out in the form of avoided heart attacks and strokes in your 70s and 80s.
5. Precision over Procrastination: Know Your Biomarkers
The future of medicine is moving away from "one size fits all." We now use specific biomarkers (like high-sensitivity CRP for inflammation or UACR for kidney health) to predict risk before a disease even shows symptoms.
The Pearl: Don't wait for a "symptom" to act. Cardiometabolic diseases are often "silent" until they are advanced. Regular screening of your lipid profile and kidney function is the only way to catch the "check engine light" before a breakdown occurs.
The Future of Cardiometabolic Health: Recent Breakthroughs in Diabetes, Heart Disease, and Obesity Management
The landscape of cardiometabolic health is experiencing a revolutionary shift. As cardiovascular disease, type 2 diabetes, and metabolic syndrome continue to burden millions globally, cutting-edge research is unveiling novel therapeutic approaches that promise to transform patient outcomes. Recent scientific literature reveals groundbreaking innovations in treating the interconnected conditions collectively known as cardiometabolic diseases—representing a paradigm shift in how we approach obesity management, cardiovascular protection, and metabolic control.
The convergence of these conditions into the cardiovascular-kidney-metabolic (CKM) syndrome framework reflects a critical understanding: these diseases don't operate in isolation. This comprehensive guide explores five landmark research publications that illuminate the path forward for cardiometabolic innovation.
Study Overview: Five Essential Research Publications
Shaping the Future of Cardiometabolic Innovation
A seminal commentary by Clemmensen et al. (2025) emphasises that obesity represents a critical intersection of cardiometabolic complications. The authors highlight recent breakthroughs in therapeutic interventions designed to counteract obesity and its cascading health risks, setting the stage for next-generation cardiometabolic medications.
The research underscores how modern obesity treatment extends beyond simple weight reduction—it addresses the underlying metabolic dysfunction that propels cardiovascular and renal complications. The authors stress the importance of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), dual and triple receptor agonists, and innovative metabolic therapeutics that simultaneously target multiple organ systems.
Diabetes Therapy: Advancing Cardiovascular, Kidney, and Metabolic Medicine
A comprehensive narrative review by Fernando et al. (2025) presents a holistic examination of the cardiovascular-kidney-metabolic syndrome framework, offering clinicians and researchers critical insights into the interconnected pathophysiology of heart disease, renal dysfunction, and metabolic disorder. The authors advocate for integrated treatment strategies that simultaneously address all three organ systems.
The review emphasizes that traditional siloed approaches—treating diabetes separately from heart disease, for example—miss crucial opportunities for synergistic therapeutic effects. Modern cardiometabolic medications increasingly demonstrate triple benefits: improvements in glucose control, cardiovascular outcomes, and renal protection. This represents a fundamental shift from organ-specific to systems-based medicine.
Key Innovations Highlighted: SGLT2 inhibitors beyond diabetes, GLP-1 agonists for cardiovascular benefits, and emerging combination therapies that enhance metabolic protection.
Novel Techniques, Biomarkers, and Molecular Targets
A new research by Di Fiore et al. (2024) emphasizes the critical role of biomarkers and molecular targets in personalizing cardiometabolic treatment. Rather than adopting a one-size-fits-all approach, the authors advocate for precision medicine strategies that identify which patients will benefit most from specific novel therapeutics.
Advanced biomarker profiling enables clinicians to detect subclinical disease stages before irreversible organ damage occurs. The study identifies emerging molecular targets such as G-protein-coupled receptors, AMPK activators, and sodium glucose cotransporter inhibitors (SGLT2i) as pivotal therapeutic pathways.
The authors stress that understanding the molecular underpinnings of cardiometabolic disease progression permits development of mechanism-based therapies that address root causes rather than merely treating symptoms. This precision approach represents the future of cardiometabolic medicine.
Expanding Novel Therapeutics Beyond Traditional Indications
Investigation by Kim (2025) explores how novel cardiometabolic medications originally developed for single-disease indications are now demonstrating extraordinary efficacy across the entire spectrum of cardiometabolic diseases. The author examines how GLP-1 receptor agonists, initially introduced for blood glucose management in type 2 diabetes, now show remarkable benefits for heart failure prevention, weight reduction, and cardiovascular event prevention.
The research illustrates a critical principle: metabolic dysfunction represents the unified pathophysiology underlying what were previously considered distinct diseases. A single medication targeting metabolic pathways can simultaneously improve outcomes across diabetes, hypertension, dyslipidemia, and cardiovascular disease.
The author highlights emerging evidence that these metabolic therapeutics may benefit patients beyond traditional "at-risk" populations, expanding treatment paradigms to include primary prevention in metabolic syndrome and pre-diabetes. This represents a fundamental reconceptualization of how we prevent chronic disease.
Novel Medications in the CKM Era
Another authoritative review by Pohlman et al. (2025) establishes the cardiovascular-kidney-metabolic syndrome as the organizing framework for modern cardiometabolic disease management. The authors comprehensively evaluate emerging cardiometabolic medications specifically designed to address all three organ systems simultaneously.
The research documents how newer agents—particularly SGLT2 inhibitors and GLP-1 agonists—demonstrate what researchers term "quadruple benefits": improved glucose control, weight reduction, blood pressure management, and cardiovascular event prevention. Some agents even show additional renal protective effects, addressing kidney disease progression.
The authors emphasize that this era requires clinicians to reframe therapeutic decision-making. Rather than selecting medications based on single outcomes (like HbA1c levels), providers must consider comprehensive cardiometabolic profiles, selecting agents that optimize outcomes across glucose metabolism, cardiovascular risk, and renal function.
Key Innovation: The emergence of triple receptor agonists (activating GLP-1, GIP, and glucagon receptors) represents the frontier of cardiometabolic therapeutics, offering unprecedented metabolic benefits.
The Science Behind Cardiometabolic Innovation
Understanding the Metabolic Dysfunction Nexus
Metabolic syndrome represents the clustering of conditions, including abdominal obesity, hypertension, dyslipidemia, and glucose intolerance. Rather than viewing these as coincidental, modern science recognizes they stem from shared underlying mechanisms: insulin resistance, mitochondrial dysfunction, and metabolic inflammation.
Obesity acts as the primary driver, creating a chronic inflammatory state that damages the endothelium, disrupts glucose handling, and promotes atherosclerosis. This understanding has revolutionized obesity management—it's no longer cosmetic but medical, addressing the root cause of multiple chronic diseases.
The GLP-1 Revolution: From Diabetes to Cardiometabolic Protection
The discovery that GLP-1 receptor agonists provide benefits beyond blood glucose control represents one of medicine's most significant recent advances. These medications act through multiple mechanisms:
Appetite suppression and satiety enhancement for sustainable weight loss
Direct cardiovascular protection through anti-inflammatory and endothelial effects
Renal protection through hemodynamic and anti-inflammatory pathways
Metabolic optimization improving lipid profiles and glucose homeostasis
This multi-system benefit pattern explains why GLP-1-based therapies dominate current cardiometabolic innovation discussions.
SGLT2 Inhibitors: Redefining Metabolic Protection
Originally developed for type 2 diabetes treatment, SGLT2 inhibitors (SGLT2i) function through a unique mechanism: blocking renal glucose reabsorption, allowing glucose excretion in urine. This elegant approach improves glucose control while reducing metabolic burden.
Remarkably, SGLT2i demonstrate cardiovascular and renal benefits even in non-diabetic populations. The research shows these medications improve heart failure outcomes, reduce chronic kidney disease progression, and lower blood pressure—effects seemingly disconnected from their glucose-lowering mechanism. This suggests SGLT2i addresses fundamental metabolic dysfunction beyond glucose regulation.
Emerging Triple Agonists: The Future is Now
The most exciting cardiometabolic innovation involves triple receptor agonists that simultaneously activate GLP-1, GIP (glucose-dependent insulinotropic polypeptide), and glucagon receptors. Early evidence suggests these agents produce superior weight reduction and metabolic benefits compared to single or dual receptor agonists.
These novel cardiometabolic medications represent the culmination of decades of basic research, offering unprecedented therapeutic power for obesity and related conditions.
The Liver: The Metabolic Hub
While the CKM framework focuses on the heart, kidneys, and blood sugar, the "Liver Pillar" incorporates MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) and MASH (Metabolic Dysfunction-Associated Steatohepatitis).
The "Spillover" Effect: A fatty liver doesn't just store fat; it becomes a source of "lipotoxic inflammation." This inflammation "spills over" into the bloodstream, directly damaging the lining of your blood vessels (endothelium) and accelerating kidney decline.
The Bidirectional Link: Research shows that as CKM stages advance, the risk of liver fibrosis (scarring) more than doubles. Conversely, the presence of liver fat is now considered a "risk enhancer" that can predict heart attacks even in young, otherwise healthy-looking individuals.
Therapeutic Synergy: This is why medications like Semaglutide and Tirzepatide are so effective; they treat the CKM syndrome by simultaneously reducing liver fat, resolving MASH inflammation, and protecting the heart.
The Pearl: Think of the liver as the "filter and furnace" of your metabolism. When the furnace is clogged with fat, it produces "toxic smoke" (inflammation) that affects every other organ in the body.
Clinical Implications: What This Means for Patients
Historically, cardiometabolic disease management focused on treating established conditions. The new paradigm emphasizes prevention through early intervention in metabolic syndrome and pre-diabetes. Patients with abdominal obesity and metabolic dysfunction now have legitimate medical reasons to pursue treatment, not for appearance but to prevent future heart disease, stroke, and kidney failure.
Personalized Medicine in Cardiometabolic Care
The emergence of biomarkers and molecular targets enables truly personalized approaches to cardiometabolic treatment. Rather than prescribing the same medication to all diabetic patients, physicians can soon match specific agents to individual metabolic profiles, optimizing outcomes.
Integrated Care Models
The cardiovascular-kidney-metabolic syndrome framework necessitates collaborative care. Cardiologists, nephrologists, and endocrinologists must communicate to select medications providing simultaneous benefits across organ systems. This integrated approach represents a fundamental reorganization of clinical medicine.
Frequently Asked Questions
Q: Can medications like GLP-1 agonists help me if I don't have diabetes? A: Yes. Recent research demonstrates cardiovascular and weight loss benefits in non-diabetic individuals. These novel therapeutics address underlying metabolic dysfunction regardless of baseline glucose levels. Consult your physician about whether you qualify.
Q: Are these new cardiometabolic medications safe for long-term use? A: Clinical trial data spanning several years show excellent safety profiles. However, like all medications, individual risks and benefits require physician assessment. The benefits for cardiovascular disease prevention appear to substantially exceed risks for most patients.
Q: How do I know if I have metabolic syndrome? A: Metabolic syndrome is diagnosed when you meet three of five criteria: abdominal obesity, elevated blood pressure, elevated fasting glucose, elevated triglycerides, and reduced HDL cholesterol. A simple blood test and measurement can determine this.
Q: What's the difference between SGLT2 inhibitors and GLP-1 agonists? A: Both improve cardiometabolic health but through different mechanisms. SGLT2i reduce blood glucose through renal glucose excretion and provide cardiovascular/renal benefits. GLP-1 agonists enhance insulin secretion, suppress appetite, and provide cardiovascular protection. Often both are used together for additive benefits.
Q: Can lifestyle changes replace these medications? A: Lifestyle modification remains foundational—diet, exercise, and weight loss directly address metabolic dysfunction. However, for many patients, cardiometabolic medications provide additional benefits that lifestyle alone cannot achieve. Think of them as complementary, not alternative.
Q: What makes these "cardiometabolic" medications different from older diabetes drugs? A: Traditional diabetes medications focused narrowly on lowering blood glucose. Modern cardiometabolic therapeutics simultaneously improve glucose control, prevent cardiovascular events, reduce kidney disease risk, and promote weight loss. This multi-system benefit represents genuine innovation.
Q: Are these medications approved by regulatory agencies? A: Yes. GLP-1 agonists and SGLT2 inhibitors carry FDA approvals. Ongoing clinical trials evaluate their use in broader patient populations and newer formulations offer improved convenience.
Q: How quickly do these medications work? A: Weight loss and appetite suppression occur relatively quickly (weeks). Cardiovascular and renal benefits typically develop over months. Optimal benefits emerge over years of consistent treatment.
Key Takeaways: The Future of Cardiometabolic Health
Paradigm Shift: Obesity and metabolic dysfunction represent root causes of multiple chronic diseases, requiring integrated treatment rather than organ-specific approaches.
Novel Therapeutics: Medications targeting GLP-1 receptors, SGLT2 transporters, and emerging triple agonists provide unprecedented benefits across glucose metabolism, cardiovascular health, and renal function.
Precision Medicine: Biomarker-driven approaches and molecular target identification enable personalized cardiometabolic treatment matching individual metabolic profiles.
Prevention Focus: Early intervention in metabolic syndrome and pre-diabetes can prevent progression to established cardiovascular disease and type 2 diabetes.
Multi-System Benefits: Modern cardiometabolic medications provide "quadruple benefits"—simultaneous improvements in glucose control, weight loss, blood pressure, and cardiovascular outcomes.
Integrated Care: The cardiovascular-kidney-metabolic syndrome framework requires collaborative approaches from multiple medical specialties.
Accessibility: As understanding grows and treatments expand, cardiometabolic innovation promises to benefit increasingly diverse patient populations beyond traditional high-risk groups.
Author’s Note
Cardiometabolic disease represents one of the most urgent and complex challenges facing modern medicine. Over the past decade, rapid advances in metabolic science, cardiovascular outcomes research, and therapeutic innovation have fundamentally altered how clinicians understand and manage conditions such as type 2 diabetes, obesity, cardiovascular disease, and chronic kidney disease. This article was written to synthesize these developments into a coherent, clinically meaningful framework grounded in the emerging concept of the cardiovascular–kidney–metabolic (CKM) syndrome
The intent is not to promote specific medications or replace individualized clinical judgment, but to highlight how contemporary evidence increasingly supports integrated, systems-based approaches over traditional organ-specific care. By examining mechanistic pathways, outcome-driven trials, and translational research, this review aims to bridge the gap between rapidly evolving science and real-world clinical practice.
All studies cited were selected for their scientific rigour, relevance to patient-centered outcomes, and contribution to advancing preventive and therapeutic strategies in cardiometabolic medicine. Where evidence continues to evolve, interpretations are presented conservatively and within the context of current consensus.
Ultimately, the goal of this article is to empower clinicians, researchers, and informed readers to think differently about cardiometabolic risk—recognizing metabolic dysfunction as a unifying driver of chronic disease and early intervention as a powerful tool for altering long-term health trajectories. As innovation accelerates, thoughtful application of evidence, combined with lifestyle optimization and personalized care, remains essential to improving population health and patient outcomes.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Obesity and Fatty Liver Disease: What Science Says About Risk and Health | DR T S DIDWAL
Intermittent Fasting: Metabolic Health Benefits and the Evidence on Longevity | DR T S DIDWAL
Activate Your Brown Fat: A New Pathway to Longevity and Metabolic Health | DR T S DIDWAL
Leptin vs. Adiponectin: How Your Fat Hormones Control Weight and Metabolic Health | DR T S DIDWAL
Lower Blood Pressure Naturally: Evidence-Based Exercise Guide for Metabolic Syndrome | DR T S DIDWAL
Movement Snacks: How VILPA Delivers Max Health Benefits in Minutes | DR T S DIDWAL
The BMI Paradox: Why "Normal Weight" People Still Get High Blood Pressure | DR T S DIDWAL
How Insulin Resistance Accelerates Cardiovascular Aging | DR T S DIDWAL
References
Clemmensen, C., Gerhart-Hines, Z., Schwartz, T. W., Zierath, J. R., & Sakamoto, K. (2025). Shaping the future of cardiometabolic innovation: advances and opportunities. Nature Metabolism, 7(8), 1495–1497. https://doi.org/10.1038/s42255-025-01343-5
Di Fiore, V., Cappelli, F., Del Punta, L., De Biase, N., Armenia, S., Maremmani, D., Lomonaco, T., Biagini, D., Lenzi, A., Mazzola, M., Tricò, D., Masi, S., Mengozzi, A., & Pugliese, N. R. (2024). Novel techniques, biomarkers and molecular targets to address cardiometabolic diseases. Journal of Clinical Medicine, 13(10), 2883. https://doi.org/10.3390/jcm13102883
Fernando, K., Connolly, D., Darcy, E., et al. (2025). Advancing cardiovascular, kidney, and metabolic medicine: A narrative review of insights and innovations for the future. Diabetes Therapy, 16(5), 1155–1176. https://doi.org/10.1007/s13300-025-01738-3
Kim, H.-J. (2025). Expanding the role of novel therapeutics in cardiometabolic syndrome: Beyond heart failure and diabetes. CardioMetabolic Syndrome Journal, 5(1), 9–22. https://doi.org/10.51789/cmsj.2025.5.e4
Pohlman, N., Patel, P. N., Essien, U. R., Tang, J. J., & Joseph, J. J. (2025). Novel cardiometabolic medications in the cardiovascular-kidney-metabolic syndrome era. The Journal of Clinical Endocrinology and Metabolism, 110(8), 2105–2122. https://doi.org/10.1210/clinem/dgaf295