Targeting Inflammaging: A Precision Strategy for Healthier Aging and Longevity
Want to live longer? Targeting inflammaging is the key. Explore how diet, exercise, and precision medicine can slow your biological clock and prevent age-related diseases today.
AGING
Dr. T.S. Didwal, M.D.(Internal Medicine)
1/23/202612 min read
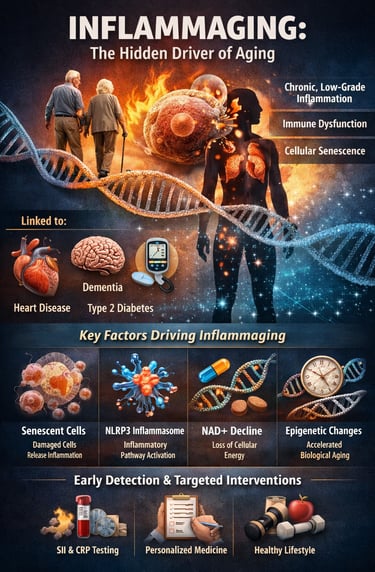
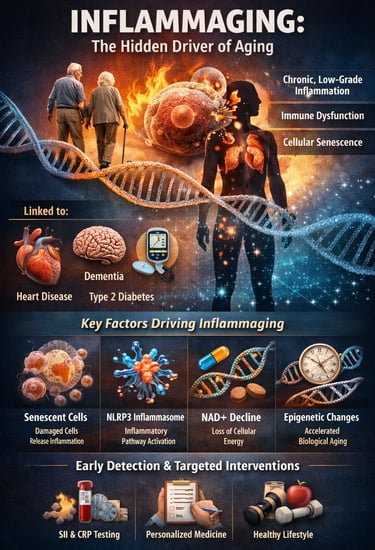
Aging is often framed as an unavoidable decline—but modern research suggests something far more powerful: how fast we age may depend heavily on chronic inflammation. Deep inside the body, a silent process called inflammaging develops over time, defined as persistent, low-grade systemic inflammation that gradually disrupts metabolism, immunity, and tissue repair (Andonian et al., 2025). Unlike short-term inflammation after injury or infection, inflammaging operates in the background for years—quietly accelerating biological wear and increasing vulnerability to disease.
What makes inflammaging so important is its strong link with cardiovascular disease, type 2 diabetes, neurodegeneration, frailty, and even reduced resilience to infections—making it a central driver of age-related chronic disease and declining healthspan (Franceschi et al., 2025). Researchers now recognize that aging is not only about time passing, but about measurable molecular changes involving immune dysregulation, immunosenescence, cellular stress pathways, and inflammatory signaling networks (Nguyen & Cho, 2025).
At the cellular level, mechanisms such as senescent cell accumulation, activation of the NLRP3 inflammasome, mitochondrial dysfunction, and declining NAD⁺ metabolism can amplify inflammatory cytokines like IL-1β and IL-6, shaping the body’s long-term inflammatory burden (Huang et al., 2025). Even more striking, emerging population-level evidence shows that simple clinical markers like the systemic immune-inflammation index (SII) may correlate with epigenetic age acceleration, suggesting routine blood tests could help detect faster biological aging early (Liu et al., 2025).
Together, these insights are shifting medicine toward precision longevity strategies—where inflammation is not just managed, but tracked, personalized, and targeted to support healthier aging trajectories.
Clinical pearls
1. The "Silent Alarm" Phenomenon
In clinical terms, inflammaging is sterile, meaning it occurs without an active infection. While acute inflammation is like a fire department responding to a blaze, inflammaging is like a faulty smoke detector that chirps 24/7. This constant "chirping" eventually wears down your cellular machinery.
The Pearl: You don’t have to "feel" sick to be experiencing systemic inflammation. Monitoring biomarkers (like hs-CRP) is essential because your body’s internal alarm system may be muffled.
2. Biological Age vs. Chronological Age
Your birth certificate tells you how many times you’ve circled the sun, but your epigenetic clock tells you how much "wear and tear" your cells have endured. Research shows that high levels of inflammation can cause your biological age to "accelerate" past your actual years.
The Pearl: Aging is a high-speed chase, but you control the accelerator. By reducing inflammatory inputs (stress, poor diet, inactivity), you can effectively "slow down" your internal clock.
3. "Zombies" in the System (Senescence)
As we age, some cells become "senescent." They stop dividing but refuse to die, lingering like "zombie cells" that secrete pro-inflammatory chemicals (known as the SASP) which "infect" healthy neighboring cells with inflammation.
The Pearl: Movement is a natural "housekeeping" tool. Regular exercise helps the immune system identify and clear out these lingering cells, preventing them from turning a localized issue into a systemic one.
4. The Power of "Immunorejuvenation"
The thymus (the "training camp" for your immune cells) naturally shrinks as we age, a process called thymic involution. This leads to a shortage of "naive" cells ready to fight new threats. However, new research suggests this isn't strictly one-way; the immune system is remarkably plastic.
The Pearl: You can "retrain" your immune system at any age. Nutrient-dense diets and specific clinical interventions act as "continuing education" for your immune cells, helping them maintain precision rather than attacking healthy tissue.
5. The Gut-Inflammation Axis
A significant portion of the body’s immune system resides in the gut. When the intestinal barrier weakens—often due to age or poor diet—inflammatory markers can leak into the bloodstream, fueling systemic inflammaging.
The Pearl: A Mediterranean-style diet isn't just about weight; it's about barrier integrity. High-fiber and polyphenol-rich foods act like "sealant" for your gut, preventing the microscopic leaks that keep your systemic inflammation levels elevated.
Inflammaging: Understanding Age-Related Inflammation and the Path to Healthier Aging
What Is Inflammaging and Why Should You Care?
Inflammaging refers to a gradual increase in systemic inflammation throughout the body as we grow older. Rather than a single disease, it's a biological process characterized by elevated levels of pro-inflammatory markers in the blood and tissues (Andonian et al., 2025). Think of it as your immune system's chronic low-grade alarm that never quite turns off.
This phenomenon affects more than just your comfort—it's increasingly recognized as a driving force behind many age-associated diseases, including cardiovascular disease, type 2 diabetes, cognitive decline, and frailty. The connection between inflammaging and these conditions has transformed how researchers and clinicians think about aging itself. Instead of viewing aging as an inevitable decline, scientists now recognize that managing inflammation and aging may be key to extending both lifespan and healthspan (the years of good health lived).
Key Takeaway
Inflammaging appears to be modifiable and potentially treatable through multi-target strategies.
The Transdisciplinary Inflammaging Framework: Connecting the Dots
In a comprehensive review published in GeroScience, Andonian and colleagues (2025) introduced an integrated transdisciplinary inflammaging framework that brings together insights from immunology, genetics, aging biology, and clinical medicine. This holistic approach recognizes that inflammaging doesn't occur in isolation—it's interconnected with multiple biological systems.
The framework highlights how immune aging (or immunosenescence) contributes to inflammaging through several mechanisms:
Accumulation of senescent immune cells that continuously produce inflammatory molecules
Dysregulation of innate immunity, causing inappropriate inflammatory responses
Reduced immune tolerance, leading the immune system to mistakenly attack healthy tissues
Thymic involution, where the thymus gland shrinks and produces fewer new immune cells
What makes this framework particularly valuable is its emphasis on precision intervention. Rather than a one-size-fits-all approach, the framework suggests that inflammaging interventions should be tailored to individual profiles—considering genetic background, lifestyle factors, existing health conditions, and biomarker profiles.
Inflammaging is multifactorial. Effective treatment requires addressing multiple biological pathways simultaneously, and personalized medicine approaches are essential for optimal outcomes.
Insights and Interventions: What Recent Research Reveals
The work of Huang, Ren, and Liu (2025) in Current Opinion in Genetics & Development provides a detailed examination of insights and interventions in age-associated inflammation. This research synthesizes current knowledge about the molecular drivers of inflammaging and explores emerging therapeutic strategies.
Their analysis reveals that several key pathways fuel the inflammaging process:
The NLRP3 inflammasome pathway appears central to inflammaging. This molecular complex acts as a sensor of cellular damage and triggers the release of inflammatory cytokines like IL-1β and IL-18. Interestingly, senescent cells—cells that have stopped dividing but remain metabolically active—are particularly good at activating this pathway.
NAD+ metabolism shows promise as a therapeutic target. NAD+ (nicotinamide adenine dinucleotide) levels naturally decline with age, and restoring them through interventions like nicotinamide riboside (NR) or pterostilbene has demonstrated anti-inflammatory effects in preclinical studies.
Cellular senescence itself offers a potential intervention point. Researchers are exploring senolytic drugs—compounds that selectively eliminate senescent cells—as a strategy to reduce chronic inflammation at its source.
Multiple molecular pathways contribute to inflammaging, and multiple intervention strategies show promise. The most effective approaches likely combine several interventions targeting different mechanisms simultaneously.
Precision Interventions and Metrics: Advancing the Field
Franceschi, Olivieri, Moskalev, and colleagues (2025) published a groundbreaking perspective in Nature Aging calling for precision interventions and metrics of inflammaging. This work addresses a critical gap: how do we measure inflammaging accurately, and how do we personalize treatment?
The authors argue that current approaches are too crude. While elevated C-reactive protein (CRP) or interleukin-6 (IL-6) can indicate inflammation, they don't capture the full picture of each person's inflammaging status. Instead, the researchers advocate for inflammaging signatures—comprehensive profiles that combine:
Systemic biomarkers: circulating inflammatory cytokines and chemokines
Cellular biomarkers: markers of immune cell aging and senescence
Functional metrics: physical performance, cognitive function, and immune responsiveness
Epigenetic markers: biological age assessments that may predict inflammaging risk
This precision approach recognizes that two people of the same chronological age may have vastly different inflammaging profiles. A 70-year-old with a low inflammaging signature might be healthier and more resilient than a 55-year-old with high inflammaging. This distinction is crucial for targeting interventions effectively.
Personalized inflammaging assessment is the future of precision medicine. One-size-fits-all aging interventions are becoming obsolete; the field is moving toward individualized risk profiling and targeted treatment.
Systemic Immune-Inflammation Index and Epigenetic Aging
Liu, Liu, Wang, Tao, and Hu (2025) conducted important research exploring the association of systemic immune-inflammation index (SII) with epigenetic age acceleration in adults using data from the National Health and Nutrition Examination Survey (NHANES). This epidemiological study reveals the real-world connections between inflammation markers and biological aging.
The SII is a composite inflammatory index calculated from platelet, neutrophil, and lymphocyte counts—readily available from standard blood tests. The researchers found that individuals with higher SII values showed evidence of epigenetic age acceleration—meaning their biological age, as measured by epigenetic clocks, exceeded their chronological age.
Epigenetic clocks are sophisticated computational models that analyze patterns of DNA methylation (chemical modifications to DNA) to estimate biological age. When someone shows epigenetic age acceleration, it suggests their cells are aging faster at a molecular level, which correlates with increased disease risk and mortality.
This connection between the SII (a simple, clinically available marker) and epigenetic aging (a sophisticated biological measurement) is significant because it suggests that routine blood tests may identify individuals at high risk for accelerated aging. This creates opportunities for early intervention—screening and treating high-SII individuals before they develop age-related diseases.
Simple inflammatory markers predict biological aging rates. Healthcare providers can now use routine lab tests to identify who needs inflammaging interventions most urgently.
Immunosenescence and Inflammaging: Targeting the Immune System
Nguyen and Cho (2025) published a comprehensive review in Experimental & Molecular Medicine specifically focused on targeting immunosenescence and inflammaging: advancing longevity research. This work emphasizes that immune system aging is central to understanding and treating inflammaging.
Immunosenescence describes the age-related decline and dysregulation of immune function. As we age, several immune changes occur:
Expansion of memory T cells at the expense of naive cells, reducing capacity to fight new infections
Reduced T cell proliferation and cytokine production
B cell dysfunction, including reduced antibody diversity
Increased production of pro-inflammatory cytokines by immune cells (the "inflammaging" phenotype)
The review highlights several intervention strategies that show particular promise:
Exercise and physical activity emerge as perhaps the most evidence-backed intervention for reducing immunosenescence. Regular aerobic exercise and resistance training help maintain immune function, reduce systemic inflammation, and improve aging outcomes—without requiring pharmaceutical intervention.
Dietary interventions, particularly those emphasizing polyphenol-rich foods (berries, leafy greens, green tea), Mediterranean-style diets, and caloric restriction or intermittent fasting, all show anti-inflammatory effects in aging populations.
Immune-modulating supplements like beta-glucans, probiotics, and certain B vitamins demonstrate potential for supporting immune function in aging adults.
Pharmaceutical approaches including ACE inhibitors (blood pressure medications) and metformin (a diabetes medication) show unexpected benefits in reducing age-associated inflammation in population studies.
Immunosenescence drives inflammaging, and multiple evidence-based interventions can slow this process. Lifestyle factors may be as powerful as pharmaceutical interventions.
Targeting LINE-1 Activation: A Novel Approach to Cardiac Aging
Yang et al. (2026) published exciting research in Nature Aging on a novel mechanism: targeting age-related LINE-1 activation alleviates cardiac aging. This study reveals a surprising connection between transposable elements and heart aging.
LINE-1 (Long Interspersed Nuclear Elements) are DNA sequences that can "jump" within the genome. In young individuals, these sequences are tightly controlled. However, with aging, LINE-1 elements become increasingly active—they mobilize more frequently, creating DNA damage and triggering inflammatory responses.
Remarkably, this LINE-1 activation specifically contributes to cardiac aging (age-related changes in the heart). The research demonstrates that preventing LINE-1 mobilization:
Reduces cardiomyocyte senescence (aging of heart muscle cells)
Decreases inflammation in cardiac tissue
Improves cardiac function and contractility
Extends cardiac lifespan in experimental models
This discovery suggests that age-related genomic instability triggered by transposable element activation is an underlying driver of tissue aging and that controlling these elements might be therapeutic. The heart appears particularly vulnerable to LINE-1 activation, though similar mechanisms likely occur in other tissues.
Controlling transposable elements may represent a novel anti-aging strategy. This research opens entirely new avenues for cardiac aging intervention and potentially extends to other age-related diseases.
Connecting the Research: An Integrated Understanding
When viewed together, these six studies paint a comprehensive picture of inflammaging:
Andonian et al. provide the conceptual framework
Huang, Ren, and Liu detail the molecular mechanisms
Franceschi et al. show how to measure and personalize treatment
Liu et al. demonstrate that routine labs can predict risk
Nguyen and Cho emphasize immunosenescence and practical interventions
Yang et al. reveal novel mechanisms like LINE-1 activation
Together, they suggest that inflammaging is:
A measurable, multifactorial process
Personalized for each individual
Preventable through multiple mechanisms
Interconnected with genomic instability, immune aging, and tissue-specific aging
Addressable through lifestyle, dietary, pharmaceutical, and potentially novel cellular interventions
Practical Implications: What This Means for You
If you're concerned about aging healthfully, consider:
Getting baseline inflammatory markers tested (CRP, IL-6, TNF-α, and CBC for SII calculation)
Engaging in regular physical activity (150+ minutes of moderate aerobic exercise weekly)
Adopting an anti-inflammatory diet emphasizing whole foods, polyphenols, and Mediterranean patterns
Managing stress through meditation, yoga, or other mindfulness practices
Ensuring quality sleep (7-9 hours nightly, as sleep deprivation increases inflammaging)
Considering periodic metabolic screening to identify aging acceleration early
Frequently Asked Questions
Q: Is inflammaging reversible? A: Yes, to a significant extent. Multiple studies show that lifestyle interventions, dietary changes, and emerging pharmacological approaches can reduce inflammatory markers and potentially reverse some aspects of immune aging. The earlier you start, the better the outcomes.
Q: How do I know if I have inflammaging? A: Simple blood tests measuring C-reactive protein (CRP), interleukin-6 (IL-6), and blood cell counts (for SII calculation) can indicate your inflammaging status. Ask your doctor for these tests, especially if you're over 50 or have chronic diseases.
Q: Are there medications that treat inflammaging? A: While no drug is specifically approved for "inflammaging," several medications used for other conditions (ACE inhibitors, metformin) show anti-inflammatory benefits. Emerging senolytic drugs and NAD+ precursors show promise but remain largely in research stages. Always consult with your doctor before starting new treatments.
Q: Can diet really affect my inflammaging status? A: Absolutely. A Mediterranean diet, emphasizing olive oil, vegetables, whole grains, legumes, and moderate fish consumption, shows particularly strong evidence for reducing systemic inflammation and aging markers. Anti-inflammatory spices like turmeric and ginger add additional benefits.
Q: What's the difference between inflammaging and normal aging? A: All aging involves some degree of physiological change. Inflammaging specifically refers to chronic, elevated inflammation that accelerates this process and increases disease risk. Normal aging can occur with minimal inflammaging, meaning chronological age and biological age can diverge significantly based on inflammatory status.
Q: Is exercise really as important as diet for reducing inflammaging? A: The research suggests they're equally important. Exercise appears uniquely capable of simultaneously reducing inflammation, improving immune function, maintaining muscle mass, and enhancing metabolic health. However, diet provides the nutritional foundation upon which exercise benefits are built.
Key Takeaways
Inflammaging is a measurable, modifiable process, not an inevitable consequence of aging
Systemic inflammation drives age-related diseases, but interventions can reduce it
Personalized assessment and treatment based on individual inflammaging profiles outperform generic approaches
Multiple intervention pathways work together: lifestyle, dietary, pharmaceutical, and novel cellular approaches
Early identification using routine labs enables preventive intervention before diseases develop
Immunosenescence is central to inflammaging, and immune function can be supported and improved
Emerging mechanisms like LINE-1 activation reveal new therapeutic targets for tissue-specific aging
The future of longevity medicine will likely be personalized, multi-targeted, and evidence-based. Rather than generic recommendations, individuals will receive tailored interventions based on their unique inflammaging signatures, genetic profiles, and existing health conditions.
Author’s Note
Aging is often described as a natural and inevitable process—but modern science is revealing that how we age is strongly influenced by biology we can measure, modify, and potentially improve. This article on inflammaging was written to bridge the gap between cutting-edge longevity research and practical health knowledge that clinicians, students, and general readers can apply.
The goal was not only to explain the mechanisms behind chronic, low-grade age-related inflammation—such as immunosenescence, cellular senescence, inflammasome activation, and epigenetic aging—but also to highlight why inflammaging matters clinically in conditions like type 2 diabetes, cardiovascular disease, frailty, cognitive decline, and reduced immune resilience.
While exciting interventions such as NAD⁺ restoration strategies, senolytic approaches, and precision biomarkers are rapidly emerging, this piece emphasizes that the strongest foundation for healthy aging still includes exercise, nutrition, sleep quality, metabolic health, and inflammation-aware lifestyle design.
This article is intended for educational purposes and reflects current scientific understanding from recent peer-reviewed literature. As research evolves, so will our ability to personalize inflammaging assessment and treatment—making longevity medicine increasingly precision-driven, evidence-based, and actionable.
References
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Exercise and Longevity: The Science of Protecting Brain and Heart Health as You Age | DR T S DIDWAL
The Science of Healthy Brain Aging: Microglia, Metabolism & Cognitive Fitness | DR T S DIDWAL
The Aging Muscle Paradox: How Senescent Cells Cause Insulin Resistance and The Strategies to Reverse It | DR T S DIDWAL
VO2 Max & Longevity: The Ultimate Guide to Living Longer | DR T S DIDWAL
Waist-Calf Ratio: The Longevity Metric Most People Aren’t Tracking | DR T S DIDWAL
Blue Zones Secrets: The 4 Pillars of Longevity for a Longer, Healthier Lifepost | DR T S DIDWAL
Anabolic Resistance: Why Muscles Age—and How to Restore Their Growth Response | DR T S DIDWAL
References
Andonian, B. J., Hippensteel, J. A., Abuabara, K., Boyle, E. M., Colbert, J. F., Devinney, M. J., Faye, A. S., Kochar, B., Lee, J., Litke, R., Nair, D., Sattui, S. E., Sheshadri, A., Sherman, A. N., Singh, N., Zhang, Y., & LaHue, S. C. (2025). Inflammation and aging-related disease: A transdisciplinary inflammaging framework. GeroScience, 47(1), 515–542. https://doi.org/10.1007/s11357-024-01364-0
Franceschi, C., Olivieri, F., Moskalev, A., et al. (2025). Toward precision interventions and metrics of inflammaging. Nature Aging, 5, 1441–1454. https://doi.org/10.1038/s43587-025-00938-7
Huang, H., Ren, J., & Liu, G. H. (2025). Insights and interventions in age-associated inflammation. Current Opinion in Genetics & Development, 91, 102306. https://doi.org/10.1016/j.gde.2024.102306
Liu, R., Liu, M., Wang, C., Tao, Z., & Hu, G. (2025). Association of systemic immune-inflammation index (SII) with epigenetic age acceleration in adults: Insights from NHANES. Epigenetics, 20(1). https://doi.org/10.1080/15592294.2025.2541248
Nguyen, T. Q. T., & Cho, K. A. (2025). Targeting immunosenescence and inflammaging: Advancing longevity research. Experimental & Molecular Medicine, 57, 1881–1892. https://doi.org/10.1038/s12276-025-01527-9
Yang, C., Du, H., Liu, S., et al. (2026). Targeting age-related LINE-1 activation alleviates cardiac aging. Nature Aging. https://doi.org/10.1038/s43587-025-01056-0