Why You Can't Lose Weight: The Chronic Inflammation & Metabolic Vicious Cycle
Struggling with weight loss? Chronic low-grade inflammation creates a metabolic block. See how diet, gut health, and lifestyle changes can reverse this vicious cycle.
OBESITY
Dr. T.S. Didwal, M.D.(Internal Medicine)
12/17/202514 min read
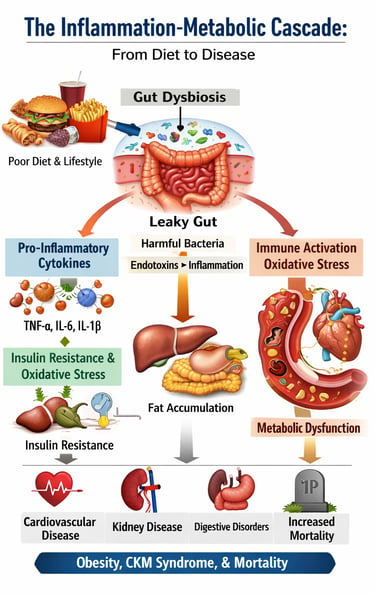
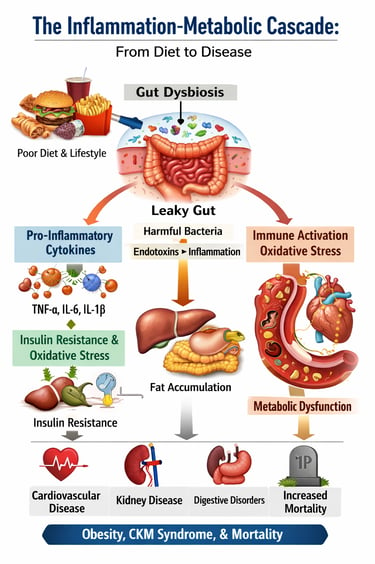
If you've ever wondered why obesity and chronic inflammation go hand in hand, or why so many people struggle with metabolic health despite their efforts, you're not alone. Recent scientific research is revealing fascinating connections between these conditions that reshape how we understand our bodies. In this comprehensive guide, we'll explore cutting-edge research on low-grade inflammation, cytokines, oxidative stress, and their profound impact on your health.
Why This Matters: Clinical Pearls
1. Inflammation Resolution is the Therapeutic Target, Not Just Suppression
The research (Soták et al., 2025) emphasizes that chronic inflammation in obesity is characterized by a failure to resolve, not just the presence of inflammatory signals.
Clinical Pearl: Interventions should move beyond simple anti-inflammatory agents (like NSAIDs) to strategies that actively support the body's natural resolution pathways (e.g., omega-3 derivatives like specialized pro-resolving mediators, or SPMs). Addressing the root cause requires restoring tissue homeostasis, not just symptom management.
2. Metabolic Health Must Be Assessed via Immune Markers
Metabolic dysfunction (insulin resistance, fatty liver) and chronic inflammation (immune cell dysfunction) are bidirectional and self-reinforcing (van de Vyver, 2023). They are inseparable disease processes.
Clinical Pearl: Clinicians should consider high-sensitivity C-reactive protein (hs-CRP) and potentially specific cytokine profiles (IL-6, TNF-alpha) as fundamental metabolic risk factors, not just markers of infection. A low-grade elevated hs-CRP in a metabolically unhealthy patient is an independent and critical indicator of mortality risk (Cao et al., 2025).
3. The Gut Microbiota is the Primary Modifiable Link to Systemic Disease
Dietary patterns (specifically insulinemic and inflammatory diets) rapidly alter the gut microbiota, which then drives systemic inflammation and metabolic dysfunction (Nepal et al., 2025).
Clinical Pearl: Therapeutic focus on the gut is paramount. Dietary modification (prioritizing fiber, prebiotics, and fermented foods) is one of the fastest ways to measurably lower systemic inflammatory biomarkers. Treat gut health as the foundational layer for managing insulin resistance and cardiovascular risk.
4. Personalized Medicine: Phenotyping by Inflammatory Clustering is Required
The "one-size-fits-all" approach to metabolic disease is insufficient because individuals cluster into distinct metabolic and inflammatory phenotypes with unique risk profiles (TuJin et al., 2025).
Clinical Pearl: Assess patients for the specific combination of their metabolic issues (glucose, lipids, blood pressure, kidney function) and their inflammatory status. This personalized metabolic-inflammatory profile dictates the most effective treatment, especially for complex conditions like digestive disorders or CKM syndrome.
5. Mitochondrial Health and Cellular Defense (Nrf2/Autophagy) are the Pharmacological Targets
At the cellular level, the chronic disease cascade is driven by oxidative stress (ROS) and a failure of protective mechanisms, specifically Nrf2 and autophagy (Michalak & Michalak, 2025; murkhuu et al., 2025).
Clinical Pearl: Prescribing medications or recommending supplements should aim to support these foundational cellular pathways (e.g., agents that promote mitochondrial biogenesis or activate the Nrf2 antioxidant defense system), not just block inflammatory signaling downstream. This validates the importance of lifestyle factors (exercise, targeted nutrients) as crucial pharmacological-level interventions.
The Hidden Connection: Understanding Chronic Inflammation, Obesity, and Metabolic Disease
Introduction: Why Chronic Inflammation Matters Now More Than Ever
Chronic low-grade inflammation—often called "inflammaging" in some contexts—has emerged as one of the most critical factors affecting modern health outcomes. Unlike acute inflammation, which helps your body fight infection, chronic inflammation operates silently in the background, contributing to obesity, metabolic dysfunction, cardiovascular disease, and even digestive disorders (Michalak & Michalak, 2025).
Recent 2025 research reveals that understanding the intricate dance between inflammatory markers, metabolic pathways, and cellular mechanisms is essential for addressing the global health crisis we face today. Let's dive into what the latest science tells us.
Inflammation and Resolution in Obesity
The comprehensive review by Soták and colleagues in Nature Reviews Endocrinology provides a landmark perspective on inflammation in obesity and, critically, the resolution of inflammation. This research emphasizes that obesity isn't simply about excess adipose tissue—it's fundamentally about dysregulated immune responses and persistent low-grade inflammation (Soták et al., 2025).
The key insight here is the concept of inflammatory resolution, which differs from merely suppressing inflammation. Instead of just reducing inflammatory signals, the body possesses sophisticated mechanisms to actively resolve inflammation, allowing tissues to return to homeostasis. When these resolution pathways fail, chronic inflammation persists.
Key Takeaways
Obesity and inflammation are intrinsically linked through shared biological pathways rather than being independent conditions
Resolution of inflammation is an active biological process, not merely the absence of inflammation
Understanding how to support inflammation resolution may be more effective than simple anti-inflammatory approaches
Immune cells in adipose tissue play a crucial role in determining whether obesity leads to chronic inflammation
Why This Matters for You
If you're struggling with weight management or metabolic health, this research suggests that simply reducing calorie intake without addressing underlying inflammatory pathways may be insufficient. Supporting your body's natural anti-inflammatory mechanisms becomes equally important.
Immunology of Chronic Low-Grade Inflammation – The Metabolic Function Connection
Van de Vyver's examination of the immunology of chronic low-grade inflammation establishes the foundational relationship between immune dysfunction and metabolic failure (van de Vyver, 2023). This research specifically explores how persistent, mild inflammation disrupts the normal functioning of metabolic tissues including liver, muscle, and adipose tissue.
The study highlights that metabolic dysfunction isn't solely about energy storage and utilization—it's deeply rooted in how the immune system perceives and responds to metabolic challenge. When immune cells become chronically activated, they release pro-inflammatory cytokines that interfere with insulin signaling, glucose metabolism, and lipid handling.
Key Takeaways
Chronic low-grade inflammation directly impairs metabolic efficiency at the cellular level
Immune cell dysfunction is both a cause and consequence of metabolic disease
The relationship between immunology and metabolism is bidirectional and self-reinforcing
Addressing metabolic function requires understanding and modulating immune responses
Why This Matters for You
If you've experienced insulin resistance, weight gain, or fatty liver disease, understanding the immune underpinnings of these conditions opens new therapeutic avenues. This isn't just about diet and exercise—it's about supporting balanced immune function.
Understanding Chronic Inflammation – The Molecular Cascade
Michalak and Michalak provide an exceptionally detailed molecular perspective on chronic inflammation, mapping the complex interactions between multiple signaling pathways (Michalak & Michalak, 2025). Their work focuses on how cytokines (inflammatory messengers), reactive oxygen species (ROS), nitric oxide (NO), and cellular stress mechanisms create an interconnected network of inflammation.
This research introduces several critical players:
Cytokines: Signaling molecules that coordinate inflammatory responses
ROS (Reactive Oxygen Species): Unstable molecules that cause oxidative stress
NO (Nitric Oxide): A regulatory molecule affecting vascular and immune function
Ca²⁺ (Calcium signaling): Intracellular messaging that triggers inflammatory cascades
HIF-1α (Hypoxia-Inducible Factor): A transcription factor activated during stress
Nrf2 (Nuclear factor erythroid 2-related factor 2): A protective antioxidant pathway
Autophagy: Cellular "cleanup" process critical for inflammation control
The coupling between these factors means that targeting single pathways is often insufficient—comprehensive approaches addressing multiple mechanisms are needed.
Key Takeaways
Chronic inflammation involves interconnected molecular pathways that amplify each other
Oxidative stress (ROS) and antioxidant defenses (Nrf2) exist in critical balance
Autophagy—cellular self-cleaning—is a crucial protective mechanism against chronic inflammation
Interventions targeting multiple pathways simultaneously may be more effective than single-target approaches
HIF-1α and calcium signaling represent critical nodes where multiple inflammatory pathways converge
Why This Matters for You
If you've heard about antioxidants, oxidative stress, or autophagy but weren't sure how they relate to your health, this research clarifies the connections. Supporting your body's natural antioxidant defenses through diet, exercise, and lifestyle choices directly impacts these molecular mechanisms.
Inflammatory Markers and Cardiovascular-Metabolic Syndrome Mortality
Cao and colleagues conducted a large prospective study examining how inflammatory markers interact with cardiovascular-kidney-metabolic (CKM) syndrome stages to predict health outcomes (Cao et al., 2025). This research moves beyond laboratory findings to real-world consequences, demonstrating that elevated inflammatory biomarkers significantly increase mortality risk, particularly when combined with metabolic dysfunction.
The study emphasizes that inflammation isn't merely an associated finding in metabolic disease—it's an independent predictor of serious health outcomes including heart disease and premature death. Moreover, the presence of multiple metabolic disorders (the CKM syndrome concept) combined with systemic inflammation creates a particularly dangerous combination.
Key Takeaways
Inflammatory markers predict all-cause mortality independent of other factors
Cardiovascular-kidney-metabolic syndrome—the co-occurrence of heart, kidney, and metabolic dysfunction—represents a high-risk state
Inflammation and metabolic dysfunction amplify each other's health impact
Early identification and management of elevated inflammatory markers can prevent serious health consequences
The joint effect of inflammation and metabolic disease is greater than either factor alone
Why This Matters for You
If you've been told you have high cholesterol, high blood pressure, diabetes, or kidney issues, this research highlights why managing inflammatory markers (like C-reactive protein) becomes a critical health priority. These factors don't operate independently—they're interconnected, and addressing them holistically is essential.
Metabolic and Inflammatory Clustering in Digestive Disease Risk
TuJin and colleagues employed advanced analytical techniques to identify distinct metabolic and inflammatory clusters—different combinations of metabolic and immune abnormalities that predict specific digestive disease risks (TuJin et al., 2025). This clustering approach reveals that people with seemingly similar metabolic profiles may have fundamentally different underlying causes of their condition.
The research demonstrates that chronic inflammation and metabolic dysfunction don't manifest uniformly across the population. Instead, distinct phenotypes (observable characteristics) emerge, each with unique risk profiles for conditions affecting the gastrointestinal tract, liver, and pancreas. Understanding which cluster a person belongs to enables more personalized medicine approaches.
Key Takeaways
Metabolic and inflammatory patterns cluster into distinct disease-risk profiles
One-size-fits-all approaches to metabolic disease miss important individual variation
Personalized medicine based on inflammatory and metabolic clustering improves risk stratification
Digestive diseases are directly linked to specific patterns of chronic inflammation and metabolic dysfunction
Identifying your specific metabolic-inflammatory profile enables targeted interventions
Why This Matters for You
If you suffer from irritable bowel syndrome, inflammatory bowel disease, fatty liver disease, or other digestive conditions, this research reveals that your specific pattern of inflammation and metabolic dysfunction likely differs from others with similar diagnoses. Precision approaches targeting your individual profile may be far more effective.
Cellular and Molecular Mechanisms – The Pharmacological Perspective
This editorial by murkhuu and colleagues synthesizes current understanding of how cellular mechanisms and molecular pathways drive metabolic disorders through inflammation and oxidative stress (murkhuu et al., 2025). The research emphasizes that pharmacological interventions must target the root causes of dysregulated inflammation and oxidative stress rather than merely treating symptoms.
The work highlights that metabolic disorders involve dysregulation at multiple cellular levels—from mitochondrial dysfunction and energy production to impaired insulin signaling and disrupted glucose metabolism. Oxidative stress (an imbalance between harmful free radicals and protective antioxidants) underpins many of these cellular disturbances.
Key Takeaways
Metabolic disorders result from dysregulation across multiple cellular systems, not single defects
Oxidative stress and inflammation are intimately connected; managing one requires addressing the other
Mitochondrial health is fundamental to preventing chronic inflammation and metabolic disease
Effective treatments must address cellular mechanisms rather than targeting inflammation in isolation
Lifestyle factors (diet, exercise, sleep, stress management) directly impact cellular health and inflammation
Why This Matters for You
If you've tried medications or supplements without lasting benefit, this research suggests that addressing underlying cellular dysfunction—through comprehensive lifestyle approaches—may be more effective than isolated interventions. Supporting your body's cellular energy systems and antioxidant defenses creates the foundation for health.
Cytokines as Key Players in Obesity and Inflammation
Uti and colleagues provide a focused examination of cytokines—the molecular messengers that orchestrate inflammation—as central players in obesity-related complications (Uti et al., 2025). Cytokines like TNF-α, IL-6, and IL-1β are released by immune cells and adipose tissue, setting off cascades that perpetuate low-grade inflammation.
What makes this research particularly valuable is its comprehensive mapping of how cytokine dysregulation in obesity leads to specific complications including insulin resistance, cardiovascular disease, and metabolic syndrome. The research demonstrates that blocking specific cytokines or reducing their production represents a promising therapeutic avenue.
Key Takeaways
Cytokines are molecular mediators linking obesity to chronic inflammation and its complications
Specific cytokine profiles identify individuals at highest risk for obesity-related complications
Pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) in obesity drive insulin resistance and metabolic dysfunction
Adipose tissue inflammation in obesity is the primary source of these damaging cytokines
Interventions reducing pro-inflammatory cytokine production show promise in reversing obesity complications
Why This Matters for You
If you have obesity or struggle with weight management, understanding that systemic inflammation driven by cytokines is your enemy—not just excess weight—reframes the approach. Reducing cytokine-driven inflammation through lifestyle modifications, specific dietary patterns, or targeted interventions may provide benefits beyond simple weight loss.
Dietary Patterns, Gut Microbiota, and Metabolic Health in Older Adults
Nepal and colleagues examined a critical link often overlooked in discussions of chronic inflammation—the role of dietary patterns in shaping gut microbiota composition and driving systemic inflammation (Nepal et al., 2025). Their research in older American men reveals that insulinemic dietary patterns (foods causing large blood sugar spikes) and inflammatory dietary patterns fundamentally alter the microbial ecosystem in the gut.
This research connects the dots between what you eat, your gut bacteria, and circulating inflammatory biomarkers. High insulinemic and inflammatory foods reduce beneficial bacteria and promote inflammatory bacterial species, which then compromise the intestinal barrier, allowing bacterial components to trigger systemic inflammation.
What This Changes in Clinical Practice
Shift from symptom control to root-cause care: Move beyond treating glucose, lipids, or weight in isolation to targeting inflammation–metabolism–gut axes.
Inflammatory markers become routine: Incorporate hs-CRP, fasting insulin, and metabolic risk staging (CKM) into standard risk assessment.
Lifestyle is first-line therapy: Prescribe anti-inflammatory dietary patterns, structured exercise, sleep optimization, and stress control as core treatments, not adjuncts.
Personalization over protocols: Recognize distinct metabolic–inflammatory phenotypes and tailor interventions accordingly.
Resolution, not suppression: Aim to restore immune balance, mitochondrial health, and autophagy, rather than relying solely on pharmacologic anti-inflammatory suppression.
This research provides tangible evidence that your dietary choices literally reshape your gut microbiota within weeks, and these changes directly impact whole-body inflammation levels. Choosing anti-inflammatory foods is simultaneously choosing beneficial gut bacteria that protect against disease.
The Unified Picture: How These Studies Connect
Looking across all eight studies, a coherent narrative emerges about chronic inflammation, obesity, and metabolic health:
The Cascade: It begins with dietary patterns and lifestyle choices that promote oxidative stress and dysbiosis. These changes trigger immune activation and elevated pro-inflammatory cytokines from both adipose tissue and gut-associated immune cells. Systemic inflammation then impairs metabolic function, leading to insulin resistance and metabolic dysfunction. As metabolic disorder progresses, inflammation worsens—creating a vicious cycle.
The Molecular Basis: At the cellular level, ROS accumulation, mitochondrial dysfunction, dysregulated calcium signaling, and impaired autophagy drive this inflammation. The failure of protective mechanisms like Nrf2 activation allows oxidative damage to persist.
The Health Consequences: Left unchecked, this inflammatory cascade manifests as obesity, cardiovascular-kidney-metabolic syndrome, digestive disease, and increased mortality risk. Conversely, understanding and interrupting any part of this cascade—through dietary modification, anti-inflammatory lifestyle changes, or targeted interventions—creates the potential for significant health improvement.
Why “Anti-Inflammatory” Monotherapy Often Fails
NSAIDs suppress symptoms, not cause: They block prostaglandins but do not resolve immune dysregulation, cytokine signaling, or metabolic inflammation, and may worsen gut permeability.
Antioxidants alone disrupt redox balance: High-dose antioxidants can blunt physiological ROS signaling, impair mitochondrial adaptation, and fail to activate endogenous systems like Nrf2.
Inflammation is network-driven: Chronic inflammation arises from cytokines, insulin resistance, gut dysbiosis, and oxidative stress acting together—single-target therapy is insufficient.
Resolution ≠ suppression: True healing requires activating inflammation-resolution pathways, autophagy, and metabolic repair, not merely blocking inflammatory signals.
Clinical takeaway: Chronic metabolic inflammation responds best to systems-level lifestyle and metabolic interventions, not isolated anti-inflammatory drugs or supplements.
Why Calorie-Only or Exercise-Only Approaches Fail
Calories ignore biology: Energy balance models do not address insulin resistance, inflammation, hormones, or circadian disruption, which drive fat storage and metabolic disease.
Exercise can’t out-train dysfunction: Without correcting diet quality, sleep, and inflammation, exercise alone yields limited and transient metabolic benefits.
Compensatory responses dominate: Calorie restriction triggers adaptive thermogenesis, hunger hormones, and lean mass loss, reducing long-term success.
Root cause is metabolic signaling, not effort: Chronic disease reflects disordered cellular signaling, not simply excess intake or insufficient activity.
Clinical takeaway: Sustainable metabolic recovery requires integrated nutrition, exercise, sleep, and inflammatory control, not isolated calorie cutting or exercise prescriptions.
Practical Applications: What You Can Do Today
Based on this research, several evidence-based strategies emerge:
1. Embrace Anti-Inflammatory Dietary Patterns
Choose whole foods that reduce insulinemic responses and support beneficial gut bacteria: colorful vegetables, whole grains, healthy fats, and fermented foods. Minimize pro-inflammatory foods like ultra-processed items, added sugars, and oxidized seed oils.
2. Support Gut Health Deliberately
Your gut microbiota is a modifiable factor. Prebiotic foods (resistant starches, high-fiber vegetables) and probiotics (fermented foods) help maintain the beneficial bacterial ecosystems that suppress systemic inflammation.
3. Address Oxidative Stress
Regular physical activity, adequate sleep, stress management, and a diet rich in antioxidants all support your body's natural antioxidant defenses including the Nrf2 pathway.
4. Optimize Metabolic Health Holistically
Weight loss is important, but supporting metabolic function through these broader approaches ensures you're addressing root causes, not just symptoms.
5. Get Biomarkers Assessed
Check your inflammatory markers (C-reactive protein, IL-6), metabolic parameters (fasting glucose, insulin, lipid profile), and, if possible, cytokine profiles to understand your specific risk profile.
Frequently Asked Questions About Chronic Inflammation and Metabolic Health
Q1: What exactly is chronic low-grade inflammation?
Chronic low-grade inflammation is a persistent, mild activation of your immune system without acute symptoms. Unlike the obvious inflammation from an infected cut or sprained ankle, low-grade inflammation operates silently, with elevated inflammatory markers like C-reactive protein circulating throughout your body. This constant immune activation damages tissues over time and drives metabolic dysfunction.
Q2: Can obesity cause inflammation, or does inflammation cause obesity?
It's bidirectional. Obesity involves enlarged adipocytes (fat cells) that secrete pro-inflammatory cytokines. This inflammation then impairs metabolic function, making weight loss harder and metabolic dysfunction worse. Breaking this cycle requires addressing both simultaneously.
Q3: How quickly can I reduce systemic inflammation?
Research shows that dietary changes can measurably reduce inflammatory markers within 4-8 weeks. Gut microbiota changes occur even faster—beneficial bacteria respond to dietary shifts within days. However, systemic changes improving metabolic function typically require 3-6 months of consistent effort.
Q4: Are anti-inflammatory supplements necessary?
While certain supplements (omega-3 fatty acids, curcumin, resveratrol) have research support, the foundation must be dietary patterns and lifestyle factors. Supplements complement but cannot replace fundamental changes to diet and activity.
Q5: Can I reduce inflammation without losing weight?
Yes. While weight loss helps, anti-inflammatory dietary patterns reduce systemic inflammation independent of weight change. Many people with metabolic dysfunction benefit significantly from inflammatory reduction before substantial weight loss occurs.
Q6: What role does exercise play in reducing inflammation?
Regular physical activity directly reduces pro-inflammatory cytokine production, improves metabolic function, supports antioxidant defenses, and promotes beneficial gut bacteria. Both aerobic exercise and resistance training provide benefits.
Q7: How do I know if I have chronic inflammation?
Standard markers include C-reactive protein (CRP), IL-6, TNF-α, and fasting insulin levels. Work with a healthcare provider to assess your personal inflammatory profile. However, even without testing, metabolic symptoms (difficulty losing weight, fatigue, brain fog, digestive issues) often indicate underlying inflammation.
Q8: Is inflammation always bad?
No. Acute inflammation is your body's essential defense mechanism. The problem is chronic, persistent inflammation that never resolves. The key is balanced immune function—appropriate responses to threats, but effective resolution returning you to bas
Conclusion: You Have More Control Than You Think
The research synthesized in this article paints a clear picture: chronic inflammation and metabolic dysfunction are not inevitable consequences of aging or genetics alone. They're responsive to modifiable factors—the food you eat, how much you move, the sleep you prioritize, and the stress you manage.
More importantly, these factors work synergistically. The anti-inflammatory diet that supports metabolic function simultaneously reshapes your gut microbiota. The exercise that improves insulin sensitivity also reduces pro-inflammatory cytokines. The sleep that supports cellular repair enables autophagy and reduces oxidative stress.
By understanding the molecular mechanisms connecting inflammation, metabolism, and health, you're equipped to make informed choices that address root causes rather than treating symptoms. The science is clear: your choices today shape your inflammatory state tomorrow, which determines your health outcomes years from now.
The time to act is now. Not because you need to be perfect, but because even modest improvements in dietary patterns, physical activity, and lifestyle quality create measurable reductions in systemic inflammation within weeks. And once inflammation decreases, your body's natural metabolic healing processes can finally begin their work.
Your health is not determined by your past choices. It's determined by your next choice—and the one after that.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Why Obesity is a Disease, Not a Lifestyle Choice: Latest Evidence-Based Insights | DR T S DIDWAL
How Insulin Resistance Accelerates Cardiovascular Aging | DR T S DIDWAL
Physical Activity, Adiposity, and Metabolic Health: What Science Reveals | DR T S DIDWAL
HIIT Benefits: Evidence for Weight Loss, Heart Health, & Mental Well-Being | DR T S DIDWAL
Sleep & Hypertension: Duration, Quality, and Blood Pressure | DR T S DIDWAL
Lower Blood Pressure Naturally: Evidence-Based Exercise Guide for Metabolic Syndrome | DR T S DIDWAL
References
Cao, Y., Wang, W., Xie, S., Xu, Y., & Lin, Z. (2025). Joint association of the inflammatory marker and cardiovascular-kidney-metabolic syndrome stages with all-cause and cardiovascular disease mortality: A national prospective study. BMC Public Health, 25(1), 10. https://doi.org/10.1186/s12889-024-21131-2
Michalak, K. P., & Michalak, A. Z. (2025). Understanding chronic inflammation: Couplings between cytokines, ROS, NO, Ca²⁺, HIF-1α, Nrf2 and autophagy. Frontiers in Immunology, 16, 1558263. https://doi.org/10.3389/fimmu.2025.1558263
murkhuu, M., Bhat, S. A., Hossain, M. Z., Shafiq, M., Hasnain, M. S., Nayak, A. K., & Ahmed, S. A. (2025). Editorial: Cellular and molecular mechanisms in metabolic disorders: Role of inflammation and oxidative stress. Frontiers in Pharmacology, 16, 1580553. https://doi.org/10.3389/fphar.2025.1580553
Nepal, S., Shi, N., Hoyd, R., Spakowicz, D. J., Orwoll, E., Shikany, J. M., & Tabung, F. K. (2025). Role of insulinemic and inflammatory dietary patterns on gut microbial composition and circulating biomarkers of metabolic health among older American men. Gut Microbes, 17(1). https://doi.org/10.1080/19490976.2025.2497400
Soták, M., Clark, M., Suur, B. E., et al. (2025). Inflammation and resolution in obesity. Nature Reviews Endocrinology, 21, 45–61. https://doi.org/10.1038/s41574-024-01047-y
TuJin, Z., Chen, Q., Zhou, L., Ye, K., Liu, Z., Jiang, W., Luo, L., Wang, Y., Ye, X., Yu, C., & Shen, Z. (2025). Integrated metabolic and inflammatory clustering reveals distinct risk profiles for digestive diseases. Advanced Science, 12(44), e11000. https://doi.org/10.1002/advs.202511000
Uti, D. E., Atangwho, I. J., Omang, W. A., Alum, E. U., Obeten, U. N., Udeozor, P. A., Agada, S. A., Bawa, I., & Ogbu, C. O. (2025). Cytokines as key players in obesity low grade inflammation and related complications. Obesity Medicine, 54, 100585. https://doi.org/10.1016/j.obmed.2025.100585
van de Vyver, M. (2023). Immunology of chronic low-grade inflammation: Relationship with metabolic function. The Journal of Endocrinology, 257(1), e220271. https://doi.org/10.1530/JOE-22-0271