Therapeutic Advances in Sarcopenia Management: From Muscle Loss to Recovery
Discover cutting-edge therapies for sarcopenia, including resistance training, nutrition strategies, SARMs, myostatin inhibitors, stem cell therapy, and precision medicine. Learn how modern science is transforming the prevention, treatment, and reversal of age-related muscle loss.
SARCOPENIA
Dr. T.S. Didwal, M.D.(Internal Medicine)
12/17/202514 min read
Your muscles aren’t just aging—they’re sending signals.
For decades, age-related muscular atrophy was dismissed as an inevitable byproduct of senescence. However, contemporary geroscience has redefined sarcopenia not merely as a loss of bulk, but as a complex myocellular signaling failure. We are currently witnessing a paradigm shift: the transition from viewing muscle as simple contractile tissue to recognizing it as a sophisticated endocrine organ that undergoes a profound molecular identity crisis with age.
At the heart of this decline is a disruption of proteostasis—the delicate equilibrium between muscle protein synthesis (MPS) and degradation.In the aging phenotype, this balance is hijacked by "inflammaging," a state of chronic, low-grade systemic inflammation characterized by elevated pro-inflammatory cytokines such as IL-6 and TNF-alpha These signals accelerate the ubiquitin-proteasome pathway, essentially "tagging" healthy muscle proteins for premature destruction.
Furthermore, the aging muscle suffers from anabolic resistance, a physiological blunting where the mTORC1 pathway—the master regulator of cell growth—fails to respond to traditional nutritional and mechanical stimuli. To override this resistance, we must move beyond general fitness advice and utilize precision interventions. By leveraging leucine-enriched amino acid kinetics, optimising mechanical mechanotransduction through progressive resistance, and targeting mitochondrial bioenergetics, we are now able to pharmacologically and behaviorally "reprogram" the aging muscle.
The following analysis explores the latest clinical advancements in reversing this neuromuscular decline, moving from traditional foundations to the frontier of regenerative medicine and biomarker-guided therapy.
Clinical Pearls
1. Exercise is the "Volume Knob" for Your DNA
Science shows that resistance training (lifting weights or using bands) does more than just build bulk; it sends a signal to your genes to stay young.
The Pearl: When you challenge your muscles, you activate a pathway called mTOR. Think of this as the "master switch" for growth.
The Action: You don't need to be a bodybuilder, but you do need "progressive overload"—gradually increasing the weight or resistance so your muscles never get too comfortable.
2. Protein Needs a "Threshold" to Work
As we age, our muscles become "hard of hearing" when it comes to protein. This is called anabolic resistance. To get the muscle to listen, you have to hit a specific amount in one sitting.
The Pearl: Eating a little protein throughout the day isn't enough. You need about 25–30 grams of protein in a single meal to "trigger" muscle building.
The Action: Focus on your breakfast. Most people get enough protein at dinner, but very little in the morning. Adding eggs or Greek yogurt to your breakfast can flip the building switch earlier in the day.
3. Leucine is the "Spark Plug"
Not all proteins are created equal. One specific amino acid called Leucine acts like a spark plug that starts the muscle-building engine.
The Pearl: Even if you eat enough protein, if it is low in Leucine, the muscle-building process (synthesis) might not start.
The Action: Prioritize "high-quality" proteins like whey, dairy, eggs, or soy. If you are plant-based, you may need to eat slightly more or look for Leucine-enriched supplements to get that "spark."
4. Vitamin D is the "Communication Wire"
You can have the best engine in the world, but it won't run if the wiring is faulty. Vitamin D acts as a bridge between your nerves and your muscles.
The Pearl: Low Vitamin D is directly linked to muscle weakness and a higher risk of falling. It helps your muscles contract faster and more efficiently.
The Action: Ask your doctor for a blood test. If you are below 30 ng/mL, your muscles are likely struggling to perform, regardless of how much you exercise.
5. "Inflammaging" is the Silent Muscle Thief
Chronic, low-grade inflammation (often called "inflammaging") acts like rust on your muscle fibers, breaking them down faster than you can repair them.
The Pearl: High levels of inflammatory markers (like CRP or IL-6) act as a signal for the body to "recycle" muscle tissue for energy.
The Action: Combat this "rust" with Omega-3 fatty acids (found in fish oil) and a diet rich in colorful vegetables. These help "cool down" the system so your exercise efforts actually stick.
What Exactly Is Sarcopenia and Why Should You Care?
Sarcopenia is the progressive loss of skeletal muscle mass, strength, and function that typically occurs with aging. Think of it as your muscles gradually "retiring" before you're ready. This isn't just about looking less toned—it's a serious medical condition that affects your independence, increases fall risk, and impacts overall quality of life.
The term comes from Greek: "sarx" (flesh) and "penia" (loss). While aging is the primary trigger, sarcopenia can strike earlier due to chronic diseases, poor nutrition, or sedentary lifestyles. Currently affecting 10-27% of adults over 60, this condition is gaining recognition as a significant public health challenge.
Understanding the Complex Biology Behind Muscle Wasting
Before we explore treatments, let's understand what's happening inside your body. According to Zheng et al. (2024) in their comprehensive review published in Archives of Pharmacological Research, sarcopenia involves a fascinating interplay of biological mechanisms.
The Molecular Players in Muscle Loss
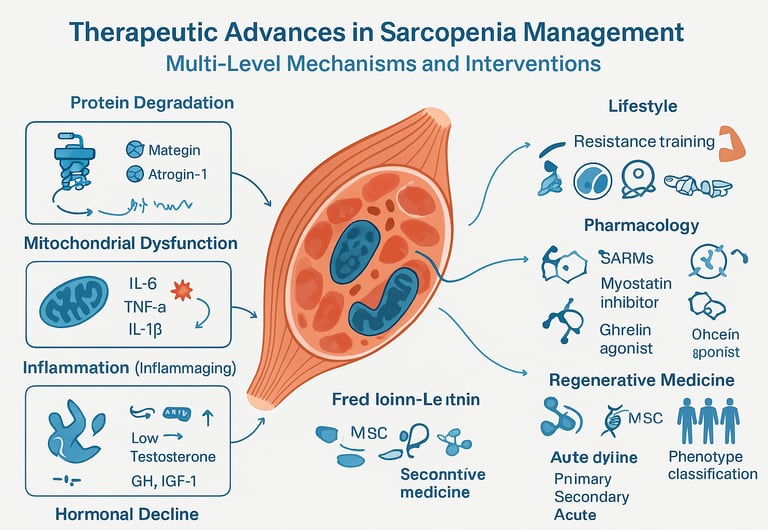
Muscle protein synthesis and degradation exist in a delicate balance. In sarcopenia, this equilibrium tips toward breakdown. Here are the key pathways involved:
Protein Degradation Pathways: The ubiquitin-proteasome system (UPS) and autophagy-lysosome pathway accelerate muscle protein breakdown. Think of these as your body's "recycling centers" working overtime. The UPS, particularly through muscle-specific E3 ubiquitin ligases like MuRF1 and atrogin-1, tags damaged proteins for destruction.
Mitochondrial Dysfunction: Your cellular powerhouses—mitochondria—become less efficient with age. Zheng's research highlights how mitochondrial damage triggers the release of reactive oxygen species (ROS), creating a vicious cycle of inflammation and further muscle degradation. This oxidative stress is like rust accumulating in your cellular machinery.
Inflammatory Cascade: Chronic low-grade inflammation, or "inflammaging," drives sarcopenia progression. Pro-inflammatory cytokines like IL-6, TNF-α, and IL-1β create a hostile environment for muscle cells. The research emphasizes how this systemic inflammation interferes with satellite cell function—your muscle's natural repair crew.
Hormonal Changes: Declining levels of anabolic hormones (testosterone, growth hormone, IGF-1) and increasing cortisol contribute significantly. These hormonal shifts reduce protein synthesis while enhancing breakdown.
Key Takeaway from Zheng et al.: Sarcopenia isn't a single pathway disorder—it's a multifactorial condition requiring comprehensive therapeutic approaches targeting multiple mechanisms simultaneously.
Traditional Interventions: The Foundation of Sarcopenia Treatment
Resistance Training: Your Muscles Best Friend
Resistance exercise remains the undisputed gold standard and cornerstone of sarcopenia management (Lo et al., 2020; Liu et al., 2025). Rather than merely being a method for maintaining fitness, progressive resistance training (PRT) is recognized as a profound physiological intervention.
The efficacy of PRT stems from its molecular action: mechanical stress directly triggers mechanotransduction—the cellular process that converts physical force into biochemical signals. This signal cascade activates key anabolic pathways, most notably the mammalian target of rapamycin (mTOR), the master regulator of muscle protein synthesis and growth.
Furthermore, PRT enhances the muscle's intrinsic repair capacity by stimulating satellite cell activation and improving neuromuscular function (Lo et al., 2020). It works at the molecular level, activating the same pathways that decline with aging, essentially "turning back the clock" on muscle cells.
Key Takeaway: Resistance training is the only intervention proven to improve all three key components of sarcopenia: mass, strength, and function, providing the essential anabolic signal that potentiates all other therapies.
Nutritional Strategies: Feeding Your Muscles Right
Kalra et al. (2025) in the Journal of Association of Physicians India provide expert consensus on nutritional management, emphasizing that diet is equally crucial as exercise.
Protein Intake Optimization: The experts recommend 1.2-1.5 g/kg body weight daily, higher than standard recommendations for older adults. But it's not just quantity—distribution matters. Consuming 25-30g of high-quality protein per meal optimizes muscle protein synthesis.
Leucine's Special Role: This branched-chain amino acid acts as a metabolic switch, triggering mTOR activation. Kalra's expert panel suggests leucine-enriched supplements (2.5-3g per meal) for those unable to meet protein needs through diet alone.
Vitamin D Supplementation: The research highlights vitamin D's dual role—supporting muscle function directly while enhancing calcium absorption for bone health. Deficiency (below 20 ng/mL) correlates strongly with sarcopenia risk.
Omega-3 Fatty Acids: EPA and DHA demonstrate anti-inflammatory properties and may enhance muscle protein synthesis, particularly when combined with exercise.
Key Takeaway from Kalra et al.: Nutritional intervention isn't optional—it's foundational. Without adequate protein and micronutrients, even the best exercise program yields suboptimal results.
Emerging Pharmacological Approaches: The New Frontier
Selective Androgen Receptor Modulators (SARMs)
Liu et al. (2025) in Clinical Nutrition provide the most current overview of pharmaceutical interventions, emphasizing the promise of SARMs. Unlike anabolic steroids, SARMs selectively target muscle and bone tissue, minimizing side effects.
These compounds activate androgen receptors in muscle cells, promoting protein synthesis without significant effects on prostate or cardiovascular systems. Clinical trials show 1-3 kg lean mass gains over 12-24 weeks. However, Liu emphasizes that regulatory approval remains pending due to long-term safety concerns.
Myostatin Inhibitors: Removing the Brakes
Myostatin, a negative regulator of muscle growth, naturally limits muscle mass. Blocking it through antibodies or inhibitors allows unrestricted muscle development. Liu's review discusses several candidates in clinical development:
Bimagrumab: Showed promising results in Phase II trials, increasing lean mass by 3.6% over 24 weeks
Landogrozumab: Under investigation with favorable early safety profiles
Key Takeaway from Liu et al.: While exciting, these therapies require careful balance—completely blocking myostatin may have unintended metabolic consequences.
Ghrelin Mimetics and Appetite Stimulation
Anamorelin and other ghrelin receptor agonists address both muscle loss and the often-accompanying cachexia. By stimulating appetite and directly promoting muscle anabolism, these dual-action agents show particular promise for cancer-associated sarcopenia.
Activin Receptor Antagonists
Najm et al. (2024) in the International Journal of Molecular Sciences highlight how activin receptor IIB (ActRIIB) antagonists block multiple negative regulators of muscle growth simultaneously—myostatin, activins, and GDFs (growth differentiation factors).
Their review notes that compounds like ACE-031 and REGN1033 demonstrated robust muscle mass increases in preclinical studies, though clinical development has faced challenges with off-target effects.
Key Takeaway from Najm et al.: Targeting upstream regulatory nodes offers more comprehensive intervention than single-pathway approaches, but requires sophisticated safety monitoring.
Regenerative Medicine: The Cutting Edge
Stem Cell Therapies
Lo et al.'s regenerative medicine perspective highlights mesenchymal stem cells (MSCs) and satellite cell therapies as potentially game-changing approaches.
Satellite Cells: These muscle-resident stem cells naturally repair damaged fibers. In sarcopenia, their number and function decline. Therapeutic strategies include:
Direct transplantation of expanded satellite cells
Enhancing endogenous satellite cell activation through growth factors
Improving the satellite cell niche through anti-inflammatory interventions
MSC Therapy: These multipotent cells can differentiate into muscle cells while secreting paracrine factors that reduce inflammation and promote regeneration. Early clinical trials show improved muscle function and reduced inflammatory markers.
Gene Therapy Approaches
Najm et al. discuss gene therapy strategies aimed at:
Overexpressing IGF-1 or follistatin (myostatin inhibitor) in muscle tissue
Delivering mitochondrial genes to restore energetic function
Using CRISPR-Cas9 to edit genes associated with muscle wasting
While mostly preclinical, these approaches represent the frontier of personalized medicine for sarcopenia.
Key Takeaway from Lo et al.: Regenerative approaches address the root cause—depleted regenerative capacity—rather than just symptoms.
Personalized Medicine: Tailoring Treatment to You
Liu et al.'s 2025 review champions the shift toward precision medicine in sarcopenia management, recognizing that one-size-fits-all approaches often fail.
Biomarker-Guided Therapy
Emerging biomarkers enable personalized treatment selection:
Inflammatory markers (CRP, IL-6): High levels suggest anti-inflammatory interventions
Hormonal profiles: Testosterone or GH deficiency indicates hormone replacement consideration
Genetic variants: Polymorphisms in myostatin, ACE, or vitamin D receptor genes influence treatment response
Circulating microRNAs: May predict sarcopenia progression and treatment efficacy
Phenotype-Based Classification
Not all sarcopenia is the same. Liu distinguishes:
Primary sarcopenia: Age-related only
Secondary sarcopenia: Disease, malnutrition, or inactivity-driven
Acute vs. chronic: Rapid loss requires urgent intervention
Each phenotype demands different therapeutic priorities.
Key Takeaway from Liu et al.: Future sarcopenia care will resemble oncology's precision approach—molecular profiling guiding individualized treatment algorithms.
Multi-Modal Interventions: Combining for Synergy
All five research papers emphasize that combination therapy outperforms monotherapy. Here's why:
Exercise + Nutrition: The Power Couple
Zheng et al. demonstrated that resistance training combined with protein supplementation yields greater muscle gains than either alone. The exercise creates anabolic signaling, while nutrition provides building blocks—a perfect synergy.
Pharmacology + Lifestyle: Enhanced Outcomes
Kalra's expert panel suggests that pharmaceutical interventions should augment, not replace, lifestyle modifications. For example, SARMs combined with resistance training might produce additive or even synergistic effects.
Addressing Comorbidities: The Holistic View
Najm et al. emphasize treating underlying conditions—diabetes, cardiovascular disease, chronic kidney disease—that accelerate sarcopenia. Optimal glucose control, cardiovascular optimization, and inflammation management create a permissive environment for muscle recovery.
Novel Therapeutic Targets on the Horizon
NAD+ Boosters and Mitochondrial Function
Zheng's review highlights NAD+ precursors (nicotinamide riboside, NMN) that restore mitochondrial function and improve muscle quality. These supplements enhance oxidative metabolism and reduce ROS production.
Senolytic Therapy
Clearing senescent cells—zombie-like cells that secrete inflammatory factors—represents an innovative approach. Dasatinib plus quercetin combination shows promise in preclinical sarcopenia models.
Microbiome Modulation
Emerging evidence suggests gut microbiota influences muscle health through:
Short-chain fatty acid production (anti-inflammatory)
Amino acid metabolism
Immune system modulation
Probiotics and prebiotics may become sarcopenia adjuncts.
Key Takeaway from Zheng et al.: Targeting aging hallmarks—mitochondrial dysfunction, cellular senescence, microbiome dysbiosis—offers novel therapeutic avenues beyond traditional muscle-centric approaches.
Practical Implementation: What This Means for You
Let's translate research into action:
For Healthcare Providers
Screen routinely: Use SARC-F questionnaire, grip strength testing, and gait speed assessment
Diagnose accurately: Employ DXA or BIA for muscle mass quantification when sarcopenia is suspected
Prescribe comprehensively: Don't just recommend exercise—provide specific programs, nutritional counseling, and consider appropriate pharmacological support
Monitor objectively: Track muscle mass, strength, and functional outcomes every 3-6 months
For Patients and Caregivers
Start early: Prevention is easier than reversal—begin resistance training in midlife
Prioritize protein: Make it the cornerstone of every meal
Stay consistent: Sporadic exercise won't work—regularity is essential
Address barriers: Pain, motivation, access—identify and solve obstacles
Consider supervised programs: Physical therapists or exercise physiologists can optimize training
Challenges and Future Directions
Research Gaps
Long-term safety data: Many novel agents lack extended follow-up
Optimal combination protocols: We don't yet know the best multi-modal regimens
Prevention vs. treatment: Most research focuses on established sarcopenia rather than prevention
Diverse populations: Studies predominantly involve European and North American cohorts
Healthcare System Barriers
Reimbursement: Many insurers don't cover sarcopenia screening or nutrition counseling
Awareness: Both public and provider recognition remains suboptimal
Access: Specialized programs aren't universally available
Najm et al. call for policy changes recognizing sarcopenia as a distinct condition deserving dedicated resources and reimbursement pathways.
Key Takeaways
🔑 Sarcopenia is multifactorial: No single intervention addresses all mechanisms—comprehensive approaches work best
🔑 Resistance training remains foundational: Nothing replaces progressive resistance exercise for building and maintaining muscle
🔑 Protein intake is critical: Older adults need more protein (1.2-1.5 g/kg/day), optimally distributed across meals
🔑 Pharmaceutical options are expanding: SARMs, myostatin inhibitors, and other agents offer promise but require careful consideration of risk-benefit profiles
🔑 Regenerative medicine represents the future: Stem cell therapies and gene therapy may revolutionize sarcopenia treatment
🔑 Personalized approaches improve outcomes: Biomarker-guided therapy and phenotype-specific treatment optimize results
🔑 Early intervention is paramount: Prevention and early treatment are far more effective than addressing advanced sarcopenia
🔑 Combination therapy is superior: Synergistic effects of exercise, nutrition, and pharmacology outperform monotherapy
Frequently Asked Questions
Q: At what age should I start worrying about sarcopenia?
A: Muscle mass begins declining around age 30, accelerating after 60. However, lifestyle modifications should begin in midlife. If you're experiencing unexplained weakness, difficulty with daily activities, or frequent falls at any age, consult your healthcare provider.
Q: Can sarcopenia be reversed completely?
A: While complete reversal to youthful levels is unlikely, significant improvements in muscle mass, strength, and function are achievable through comprehensive intervention. Early-stage sarcopenia responds better than advanced disease. The research by Liu et al. shows that personalized, multi-modal approaches can restore functional independence in many cases.
Q: How quickly will I see results from treatment?
A: Muscle strength improvements appear within 4-8 weeks of resistance training, while measurable muscle mass gains typically require 12-16 weeks. Functional improvements (walking speed, stair climbing) often manifest within 6-8 weeks. Consistency is crucial—intermittent efforts yield minimal benefits.
Q: Is sarcopenia only an aging problem?
A: While aging is the primary risk factor, secondary sarcopenia occurs in younger individuals with chronic diseases (cancer, heart failure, kidney disease), prolonged immobilization, or severe malnutrition. Kalra et al. emphasize that any prolonged catabolic state can trigger muscle wasting regardless of age.
Q: Are supplements necessary or can diet alone provide sufficient protein?
A: For most individuals, whole-food sources provide adequate protein intake. However, older adults with poor appetite, dietary restrictions, or very high protein needs may benefit from supplementation. Whey protein, particularly leucine-rich formulations, offers convenient, high-quality options. Consult a dietitian for personalized recommendations.
Q: Do I need to lift heavy weights, or will light exercise work?
A: Research, including work by Lo et al. (2020), consistently demonstrates that progressive overload achieved via high-intensity resistance training (70–85% of $1\text{RM}$) is required for superior hypertrophic and strength outcomes."
Q: What about testosterone replacement therapy?
A: Hormone replacement can benefit individuals with confirmed hypogonadism (low testosterone), but isn't appropriate for everyone. Zheng et al. note that testosterone increases muscle mass but carries cardiovascular and prostate risks. Discuss risks and benefits thoroughly with your physician, and consider it only within comprehensive management programs.
Q: How does sarcopenia differ from cachexia?
A: While both involve muscle loss, cachexia is a complex metabolic syndrome associated with underlying illness (cancer, heart failure), characterized by inflammation, anorexia, and involuntary weight loss. Sarcopenia primarily involves age-related muscle changes without necessarily causing overall weight loss. Treatment approaches overlap but cachexia requires addressing the underlying disease.
Q: Are there any foods that specifically help build muscle?
A: No single "superfood" exists, but leucine-rich protein sources (dairy, eggs, meat, fish, soy) optimally stimulate muscle protein synthesis. Vitamin D-rich foods (fatty fish, fortified dairy), omega-3 sources (salmon, walnuts, flaxseed), and antioxidant-rich fruits and vegetables support overall muscle health by reducing inflammation and oxidative stress.
Q: Will walking or cardio exercise help with sarcopenia?
A: Aerobic exercise improves cardiovascular health, mobility, and endurance but doesn't significantly increase muscle mass or strength. It's an important component of overall health but must be combined with resistance training for sarcopenia management. Najm et al. recommend balanced programs incorporating both modalities.
The Bottom Line
Sarcopenia is no longer viewed as an inevitable consequence of aging but as a modifiable, multifactorial biological disorder driven by impaired muscle signaling, anabolic resistance, mitochondrial dysfunction, and chronic low-grade inflammation. Modern research shows that muscle functions as an endocrine and metabolic organ, making early and targeted intervention critical for preserving strength, mobility, and independence.
Progressive resistance training remains the cornerstone of therapy, providing the primary anabolic signal required to restore muscle mass, strength, and function. This effect is potentiated by adequate protein intake (1.2–1.5 g/kg/day), optimized meal-wise distribution, and leucine enrichment, which help overcome age-related anabolic resistance. Correction of vitamin D deficiency and adequate omega-3 fatty acid intake further enhance neuromuscular function and reduce inflammation.
Pharmacological therapies such as SARMs, myostatin inhibitors, ghrelin mimetics, and activin receptor antagonists show promise but remain largely investigational and should complement—not replace—lifestyle interventions. Regenerative and gene-based therapies represent future avenues but are currently limited to research settings.
The future of sarcopenia care lies in precision medicine, integrating biomarkers, phenotype classification, and multimodal strategies. Early identification and combined interventions offer the greatest potential to prevent disability and restore functional independence in aging populations.
Disclaimer: This article provides educational information based on recent scientific research and should not replace professional medical advice. Always consult qualified healthcare providers before starting new treatments, exercise programs, or supplements.
Emerging therapies discussed in this article—such as Selective Androgen Receptor Modulators (SARMs), myostatin/activin inhibitors, gene therapy approaches, and stem cell–based interventions—are investigational in nature. Most are not approved for routine clinical use, remain limited to clinical trials, or are subject to strict regulatory oversight due to incomplete long-term safety and efficacy data. Their use outside approved research settings may carry significant medical, ethical, and legal risks. These therapies should not be used as substitutes for established interventions such as resistance training, adequate nutrition, and management of underlying disease. Any consideration of advanced or experimental treatments must occur only within regulated clinical trials or under specialist supervision, in accordance with national and international guidelines.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
How to Prevent and Reverse Muscle Wasting in Chronic Disease (2025 Guide) | DR T S DIDWAL
Vitamin D Deficiency and Sarcopenia: The Critical Connection | DR T S DIDWAL
How to Prevent Sarcopenia: Fight Age-Related Muscle Loss and Stay Strong | DR T S DIDWAL
Who Gets Sarcopenia? Key Risk Factors & High-Risk Groups Explained | DR T S DIDWAL
Sarcopenia: The Complete Guide to Age-Related Muscle Loss and How to Fight It | DR T S DIDWAL
Best Exercises for Sarcopenia: Strength Training Guide for Older Adults | DR T S DIDWAL
Vitamin D Deficiency and Sarcopenia: The Critical Connection | DR T S DIDWAL
References:
Liu, X., Chen, X., & Cui, J. (2025). Therapeutic advances in sarcopenia management: From traditional interventions to personalized medicine. Clinical Nutrition, 51, 187–197.
Kalra, S., Das, A., Kotwal, N., et al. (2025). Expert Opinion on Management Advancements in Sarcopenia: From Muscle Wasting to Recovery. Journal of Association of Physicians India, 73(6), 50-60.
Zheng, Y., Feng, J., Yu, Y., et al. (2024). Advances in sarcopenia: mechanisms, therapeutic targets, and intervention strategies. Archives of Pharmacological Research, 47, 301–324.
Lo, J. H., U, K. P., Yiu, T., Ong, M. T., & Lee, W. Y. (2020). Sarcopenia: Current treatments and new regenerative therapeutic approaches. Journal of Orthopaedic Translation, 23, 38–52.
Najm, A., Niculescu, A. G., Grumezescu, A. M., & Beuran, M. (2024). Emerging therapeutic strategies in sarcopenia: An updated review on pathogenesis and treatment advances. International Journal of Molecular Sciences, 25(8), 4300. https://doi.org/10.3390/ijms25084300