Testosterone and Longevity in Aging Men: How Hormonal Health Shapes Metabolism, Muscle, and Vitality
Unlock the connection between hormonal optimization and life extension. This evidence-based guide explores the critical link between testosterone and metabolic vitality, synthesizing 2024–2025 clinical data on TRT, PADAM, and long-term health outcomes for aging men.
AGING
Dr. T.S. Didwal, M.D.(Internal Medicine)
12/23/202511 min read

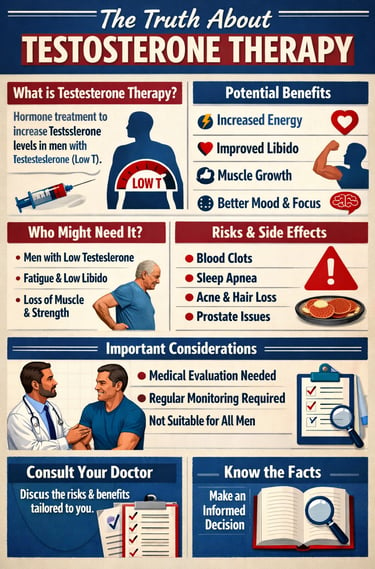
Hypogonadism, or low testosterone, affects millions of men worldwide and is increasingly recognized as a significant public health concern. From metabolic dysfunction to reproductive challenges, the consequences of androgen deficiency extend far beyond sexual dysfunction
In the evolving landscape of geroscience, Partial Androgen Deficiency in Aging Men (PADAM) is no longer viewed as a mere byproduct of chronological aging, but as a primary driver of multisystem senescence. The transition from physiological "wellness" to "frailty" is frequently paved by a gradual decline in serum testosterone, which serves as a master regulator of metabolic homeostasis. Recent landmark syntheses, including the Cruickshank et al. (2024) TestES evidence review, have fundamentally reframed Testosterone Replacement Therapy (TRT) from a quality-of-life intervention to a cornerstone of chronic disease prevention.
The link between androgens and longevity is anchored in the mitochondrial and metabolic axis. Androgen deficiency is not just a symptom of metabolic syndrome; it is a pathogenic catalyst for insulin resistance, visceral adiposity, and pro-inflammatory cytokine signaling. By stabilizing the androgenic environment, clinicians can potentially arrest the "metabolic cascade" that leads to cardiovascular compromise and neurodegenerative decline. As we navigate the clinical insights of 2025, it becomes clear that restoring hormonal equilibrium is not about "turning back the clock"—it is about optimizing the biological machinery to ensure that vitality remains commensurate with longevity.
Clinical Pearls
1. The "Dawn" Requirement for Diagnosis
Biological rhythms matter. Testosterone levels naturally peak in the early morning (typically between 7:00 AM and 10:00 AM). To avoid a "false positive" diagnosis of hypogonadism, clinical guidelines require at least two separate morning blood draws. If you test in the afternoon, your levels may appear artificially low, leading to unnecessary treatment.
2. TRT is a "Metabolic Multiplier," Not a Magic Bullet
While testosterone replacement therapy (TRT) is highly effective at improving insulin sensitivity and reducing visceral (belly) fat, it works best as a partner to lifestyle changes. Think of TRT as a "metabolic multiplier"—it provides the energy and physiological environment to make exercise and nutrition more effective, but it cannot fully replace them.
3. The Fertility "Fork in the Road"
Standard TRT acts as a male contraceptive by signaling the brain to stop producing the hormones (FSH and LH) that drive sperm production. For men who wish to maintain fertility, Clomiphene Citrate is often the preferred path. It "tricks" the brain into boosting your own natural production rather than replacing it with an external source.
4. The "Symptom Timeline" Expectations
Hormonal recovery is a marathon, not a sprint. While improvements in mood and sexual desire often appear within the first 3 to 6 weeks, structural changes—such as increased muscle mass, bone density, and significant metabolic shifts—typically require 6 to 12 months of consistent optimization. Patience is a clinical necessity.
5. The Prostate "Saturation" Theory
Modern evidence has shifted the perspective on prostate health. We now understand that once testosterone reaches a certain (relatively low) level, the prostate receptors become "saturated." Beyond this point, supplemental testosterone does not appear to "fuel" prostate cancer. However, regular monitoring of PSA (Prostate-Specific Antigen) remains essential to ensure any underlying issues are caught early.
Testosterone Replacement Therapy for Hypogonadism: Evidence-Based Strategies and Clinical Insights
Understanding Hypogonadism: Pathogenesis, Diagnosis, and Comprehensive Management
Hypogonadism is characterized by insufficient testosterone production or action, leading to numerous physiological complications. The condition manifests differently depending on age, severity, and underlying causes. Understanding this distinction is crucial for tailored treatment approaches.
A landmark review by de Silva et al. (2024) in The Lancet Diabetes & Endocrinology provides comprehensive insights into male hypogonadism pathogenesis, diagnosis, and management strategies. This authoritative source synthesizes current understanding of how hypogonadism develops, establishes diagnostic criteria and best practices, and outlines evidence-based management pathways. The review emphasizes that effective hypogonadism management begins with accurate diagnosis and understanding underlying causes—whether primary testicular dysfunction, secondary central hypogonadism, or age-related androgen deficiency. By clarifying the pathophysiological mechanisms driving hypogonadism, clinicians can select appropriate interventions and predict treatment response more reliably.
The Role of PADAM in Chronic Disease Development
Partial androgen deficiency of aging men (PADAM) represents a natural but problematic decline in testosterone levels occurring in mature male populations. Unlike primary hypogonadism, which involves testicular failure, PADAM develops gradually and is often underdiagnosed.
According to Pechersky (2015), PADAM plays a pivotal role in developing metabolic syndrome—a cluster of conditions including obesity, insulin resistance, hypertension, and dyslipidemia. The research reveals that androgen deficiency doesn't merely coincide with metabolic dysfunction; it actively contributes to its pathogenesis. This finding fundamentally shifts our understanding of aging men's health, suggesting that testosterone replacement might address not just symptoms but underlying metabolic disease.
The implications are profound: by treating androgen deficiency, clinicians may simultaneously address multiple components of metabolic syndrome, potentially reducing cardiovascular risk and improving overall metabolic health in aging populations.
Testosterone Replacement Therapy: Safety, Efficacy, and Real-World Evidence
Comprehensive Evidence Synthesis: The TestES Evaluation
One of the most significant recent contributions to the TRT literature comes from a major evidence synthesis by Cruickshank et al. (2024). This extensive review, published in the Health Technology Assessment journal as the TestES evidence synthesis, systematically evaluated the effects and safety of testosterone replacement therapy in men with confirmed hypogonadism through rigorous systematic review methodology.
The Cruickshank team conducted comprehensive literature synthesis examining randomized controlled trials, observational studies, and real-world evidence to establish definitive conclusions about TRT efficacy and safety. Their systematic approach evaluated diverse patient populations across varying age groups, baseline health statuses, and treatment durations, ensuring findings apply broadly to clinical practice rather than narrow study populations.
Contrary to earlier concerns, the researchers' rigorous analysis of TRT safety profiles demonstrated that testosterone replacement therapy carries acceptable safety profiles when appropriately administered to confirmed hypogonadal men. Examination of cardiovascular outcomes revealed nuanced findings—rather than uniformly increasing risk, cardiovascular responses depend substantially on baseline health status, concurrent comorbidities, and baseline cardiovascular risk factors. Men with well-controlled hypertension and appropriate baseline cardiovascular assessment tolerated testosterone treatment well, while those with untreated cardiovascular disease required careful consideration.
Prostate Health Considerations:
Regarding prostate safety—historically the primary concern limiting testosterone therapy—Cruickshank et al. found that testosterone replacement neither increases prostate cancer incidence nor accelerates benign prostatic hyperplasia progression in most patients. However, men with existing prostate disease or elevated baseline prostate-specific antigen required individualized assessment and monitoring rather than categorical exclusion from treatment.
Metabolic and Sexual Function Benefits:
The synthesis documented consistent improvements across multiple domains. Sexual function restoration occurred relatively rapidly following treatment initiation, with improvements in erectile function, libido, and sexual satisfaction evident within 6-12 weeks. Beyond sexual outcomes, testosterone treatment produced measurable improvements in metabolic parameters, body composition, bone density, and mood scores. Mood improvements, while significant, developed more gradually than sexual function improvements, suggesting different mechanisms and timelines for neuropsychiatric versus sexual benefits.
Economic Evaluation Findings:
A distinctive contribution of the Cruickshank analysis involved comprehensive economic evaluation alongside clinical assessment. The cost-effectiveness analysis suggested that testosterone replacement therapy may reduce overall healthcare expenditure by preventing complications associated with untreated hypogonadism—including cardiovascular events, bone fractures, metabolic syndrome progression, and reduced work productivity. This economic dimension strengthens the case for evidence-based hypogonadism treatment, demonstrating value beyond symptom relief.
Clinical Implications:
For clinicians and patients alike, the TestES synthesis offers robust evidence-based guidance: TRT can be safely administered when initiated in patients with confirmed androgen deficiency through appropriate diagnostic protocols, comprehensive baseline assessment including cardiovascular and prostate evaluation, and adherence to evidence-based monitoring protocols. The synthesis emphasizes that safety concerns should not categorically exclude appropriate candidates, but rather guide careful patient selection and individualized monitoring strategies.
Recent Clinical Insights (2025)
Goulis (2025) provides updated perspective on hypogonadism and testosterone replacement therapy, synthesizing lessons from recent clinical trials and observational studies. This contemporary analysis emphasizes personalized medicine approaches, recognizing that optimal TRT strategies vary based on patient age, comorbidities, and baseline health status.
The evidence demonstrates that testosterone replacement benefits extend across multiple domains: sexual function improves within weeks, while metabolic and cardiovascular benefits may take months to manifest. Goulis highlights the importance of patient selection and monitoring, noting that TRT efficacy depends not merely on hormone administration but on comprehensive management including lifestyle optimization and comorbidity control.
Beyond Traditional TRT: Clomiphene Citrate as an Evidence-Based Alternative
Fertility-Preserving Treatment in Male Hypogonadism
While testosterone replacement therapy remains the gold standard for most hypogonadism cases, clomiphene citrate offers a compelling alternative, particularly for men concerned with fertility preservation.
Câmara (2025) present evidence-based prescription strategies for clomiphene citrate in male hypogonadism and fertility management. Unlike exogenous testosterone, which suppresses endogenous testosterone production, clomiphene citrate works by blocking estrogen feedback at the hypothalamic-pituitary axis, stimulating the body's own testosterone production.
The distinct advantages of clomiphene therapy include:
Fertility preservation represents the primary advantage over conventional testosterone replacement. Men receiving clomiphene citrate maintain spermatogenesis, making this approach ideal for younger men or those planning families. Additionally, clomiphene treatment often avoids the systemic hormonal disruption associated with exogenous testosterone administration.
Câmara's research emphasizes that clomiphene citrate prescription requires careful patient selection, appropriate dosing protocols, and monitoring for efficacy and tolerability. For select populations—particularly younger men with secondary hypogonadism—clomiphene-based therapy provides an evidence-supported alternative worth considering before initiating testosterone replacement.
The Broader Health Impact: Hypogonadism and Prostate Disease
PADAM's Role in Prostate Pathology
Pechersky (2025) extends prior work by examining the influence of partial androgen deficiency in aging men on benign prostatic hyperplasia and prostate cancer development. This investigation reveals complex relationships between testosterone levels and prostate health.
The findings suggest a nuanced picture: while androgens are essential for prostate function, the relationship between androgen deficiency and prostate cancer follows a "sweet spot" theory—both deficiency and excess may carry risks. This understanding complicates simplistic narratives about testosterone replacement and cancer risk, emphasizing the necessity for individualized assessment and monitoring.
For men with existing prostate disease, testosterone replacement therapy requires careful consideration and baseline prostate-specific antigen assessment. The evidence supports cautious optimization of testosterone levels rather than avoiding treatment entirely, provided appropriate screening and surveillance occur.
Clinical Decision-Making: Integrating Evidence into Practice
Assessment and Baseline Evaluation
Before initiating any hypogonadism treatment, comprehensive baseline assessment is essential. Standard practice includes:
Confirming low testosterone levels with appropriate laboratory testing, recognizing that testosterone measurements require careful timing (early morning) and may need repetition for diagnosis. Evaluating metabolic health markers including glucose metabolism, lipid profiles, and blood pressure. Assessing cardiovascular risk through standard risk stratification tools. Screening for prostate disease with digital rectal examination and prostate-specific antigen levels when appropriate.
Treatment Selection
The choice between testosterone replacement therapy and clomiphene citrate depends on multiple factors. Testosterone replacement suits most men with confirmed hypogonadism, particularly those with primary testicular disease or who've completed family planning. Clomiphene citrate serves younger men, those desiring fertility preservation, or those with secondary hypogonadism where endogenous production might respond to stimulation.
Ongoing Monitoring
Effective hypogonadism management requires regular follow-up including testosterone level monitoring (typically 6-8 weeks after treatment initiation), cardiovascular risk assessment, prostate cancer screening continuation, and symptom evaluation.
Key Takeaways
Male hypogonadism pathogenesis varies significantly based on whether dysfunction originates from primary testicular failure or secondary central hypogonadism, necessitating differentiated diagnostic and treatment approaches as outlined in current clinical guidelines.
PADAM significantly contributes to metabolic syndrome development in aging men, suggesting that androgen deficiency treatment addresses root causes rather than merely managing symptoms.
Testosterone replacement therapy demonstrates safety and efficacy in appropriately selected patients with confirmed hypogonadism, with benefits spanning sexual function, metabolic health, and physical capacity.
Clomiphene citrate offers a fertility-preserving alternative to exogenous testosterone, making it ideal for specific patient populations concerned with reproductive capability.
The relationship between hypogonadism and prostate disease is complex, requiring individualized assessment and monitoring rather than categorical restrictions on testosterone treatment.
Accurate hypogonadism diagnosis requires understanding pathophysiological mechanisms and employing appropriate diagnostic protocols before initiating treatment, preventing unnecessary therapy while ensuring symptomatic individuals receive needed care.
Evidence-based hypogonadism management requires comprehensive baseline assessment, appropriate treatment selection, and regular monitoring protocols.
Recent research (2024-2025) emphasizes personalized medicine approaches to testosterone replacement therapy, recognizing individual variation in response and requirements based on hypogonadism type and etiology.
Frequently Asked Questions
Q: How do clinicians differentiate between primary and secondary hypogonadism?
A: According to de Silva et al. (2024), hypogonadism diagnosis requires understanding pathophysiological mechanisms. Primary hypogonadism involves testicular dysfunction (elevated luteinizing hormone), while secondary hypogonadism reflects central nervous system dysfunction (low luteinizing hormone). This distinction fundamentally guides treatment selection and prognosis.
Q: Is testosterone replacement therapy safe for long-term use?
A: Current evidence, particularly from Cruickshank et al. (2024), supports testosterone replacement therapy safety in appropriately selected patients with regular monitoring. Safety depends on patient selection, baseline health status, and adherence to monitoring protocols rather than the treatment itself being inherently unsafe.
Q: Can testosterone replacement therapy cause prostate cancer?
A: The relationship is complex and nuanced. While androgens are necessary for prostate function, neither extremely low nor excessively high testosterone levels represent optimal targets. Pechersky (2025) suggests monitoring rather than categorical avoidance of testosterone treatment in men at risk.
Q: Is clomiphene citrate as effective as testosterone replacement?
A: Clomiphene citrate effectively stimulates endogenous testosterone production in secondary hypogonadism, but may be less effective in primary testicular disease. The choice depends on diagnosis type and individual patient goals, particularly fertility considerations.
Q: How long before experiencing benefits from testosterone treatment?
A: Sexual function improvements typically appear within weeks, while metabolic and body composition changes require months. Timeline varies individually based on baseline status and treatment type.
Q: What happens if I stop testosterone replacement therapy?
A: Exogenous testosterone replacement suppresses endogenous production. Discontinuation results in return to baseline hypogonadal states unless underlying disease resolved. Clomiphene citrate may allow continued endogenous production after discontinuation.
Call to Action
If you're experiencing symptoms of male hypogonadism—including fatigue, reduced libido, mood changes, or metabolic dysfunction—don't assume these are inevitable consequences of aging. Recent research provides compelling evidence that evidence-based hypogonadism treatment can significantly improve quality of life and reduce disease risk.
Take these next steps:
Schedule a comprehensive evaluation with your healthcare provider, requesting testosterone level testing and full metabolic assessment.
Discuss treatment options openly, exploring both testosterone replacement therapy and clomiphene citrate approaches based on your health status and personal goals.
Commit to monitoring protocols, understanding that effective hypogonadism management requires partnership with your healthcare team through regular follow-up.
Explore lifestyle optimization simultaneously—exercise, nutrition, sleep, and stress management amplify testosterone treatment benefits.
Stay informed about emerging evidence, as the field of hypogonadism treatment continues evolving with new research annually.
Your hormonal health matters. With evidence-based approaches, appropriate medical guidance, and commitment to comprehensive management, hypogonadism treatment can restore vitality, metabolic function, and quality of life.
Medical Disclaimer
This article is provided for informational and educational purposes only and does not constitute medical advice, diagnosis, or treatment. Testosterone Replacement Therapy (TRT) and clomiphene citrate are prescription interventions that carry potential risks and side effects. Always seek the advice of a qualified healthcare professional regarding any medical condition or before initiating new hormonal therapies. Never disregard professional medical advice or delay seeking it because of something you have read in this article. The synthesis of 2024–2025 research reflects current clinical trends but should be interpreted within the context of your unique medical history.
Related Articles
Hormone Therapy and Sarcopenia: Testosterone, HGH, and Muscle Mass | DR T S DIDWAL
How to Prevent Sarcopenia: Fight Age-Related Muscle Loss and Stay Strong | DR T S DIDWAL
Best Supplements for Sarcopenia: Vitamin D, Creatine, and HMB Explained | DR T S DIDWAL
Testosterone Therapy for Sarcopenia: A Comprehensive Evidence-Based Guide | DR T S DIDWAL
References
Câmara, L. C. (2025). Evidence-based prescription strategies for clomiphene citrate in male hypogonadism and fertility management. Asian Journal of Research in Medical and Pharmaceutical Sciences, 14(1), 1–7. https://doi.org/10.9734/ajrimps/2025/v14i1289
Cruickshank, M., Hudson, J., Hernández, R., Aceves-Martins, M., Quinton, R., Gillies, K., Aucott, L. S., Kennedy, C., Manson, P., Oliver, N., Wu, F., Bhattacharya, S., Dhillo, W. S., Jayasena, C. N., & Brazzelli, M. (2024). The effects and safety of testosterone replacement therapy for men with hypogonadism: The TestES evidence synthesis and economic evaluation. Health Technology Assessment (Winchester, England), 28(43), 1–210. https://doi.org/10.3310/JRYT3981
Goulis, D. (2025). Hypogonadism and testosterone replacement: What did we learn from the recent studies? Maturitas, 199, 108369. https://doi.org/10.1016/j.maturitas.2025.108369
Pechersky, A. V. (2015). Role of partial androgen deficiency of aging men in development of the metabolic syndrome. American Research Journal of Urology, 1(1), 10–22. https://www.arjonline.org/papers/arju/v1-i1/2.pdf
Pechersky, A. V. (2025). The influence of partial androgen deficiency in aging men (PADAM) on the development of benign prostatic hyperplasia and prostate cancer. American Research Journal of Urology, 3(1), 1–16. https://www.arjonline.org/papers/arju/v3-i1/1.pdf
de Silva, N. L., Papanikolaou, N., Grossmann, M., Antonio, L., Quinton, R., Anawalt, B. D., & Jayasena, C. N. (2024). Male hypogonadism: Pathogenesis, diagnosis, and management. The Lancet Diabetes & Endocrinology, 12(10), 761–774. https://doi.org/10.1016/S2213-8587(24)00199-2