Is Testosterone Replacement Therapy Safe for Your Heart? Insights from the 2026 TRAVERSE Trial and Recent Meta-Analyses
Review 2026 evidence on testosterone replacement therapy and cardiovascular outcomes, including MACE data, meta-analyses, and expert panel recommendations.
HEART
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/14/202612 min read
For decades, testosterone replacement therapy (TRT) has lived under a cloud of cardiovascular uncertainty. In the early 2010s, observational studies suggested a possible increase in myocardial infarction and stroke among men prescribed testosterone, prompting regulatory warnings and widespread clinical caution. Prescriptions declined, headlines amplified concern, and many physicians hesitated—even when treating men with clear biochemical hypogonadism. The central question lingered: Does restoring testosterone protect the aging male phenotype—or endanger the heart?
Fast forward to 2026, and the evidence landscape looks markedly different. The landmark TRAVERSE trial, specifically designed to evaluate major adverse cardiovascular events (MACE: cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke), demonstrated non-inferiority of testosterone therapy compared with placebo in appropriately screened men (Zitzmann et al., 2026). Complementary meta-analyses of randomized controlled trials have likewise found no significant increase in cardiovascular events when TRT is prescribed using physiologic dosing and careful monitoring (Abdelaziz, 2025). Meanwhile, contemporary endocrine analyses emphasize that endogenous testosterone deficiency itself is associated with adverse cardiometabolic profiles, complicating the simplistic narrative that “testosterone equals risk” (Yeap & Anawalt, 2026).
Yet reassurance should not be mistaken for complacency. Testosterone stimulates erythropoiesis, may influence fluid balance, and interacts with underlying cardiovascular disease in complex ways. Patient selection, hematocrit monitoring, formulation choice, and baseline cardiac status remain critical determinants of safety. The modern debate is no longer whether testosterone is inherently dangerous—but rather in whom, under what conditions, and at what dose it can be used safely.
Understanding what the latest trials truly show—and what uncertainties remain—is essential for both clinicians and patients navigating TRT decisions in 2026.
Clinical pearls.
1. The "Physiologic Range" Guardrail
Therapeutic success in TRT is defined by the restoration of serum testosterone to mid-physiologic levels 350--700 ng/dL, as supraphysiologic peaks are associated with increased erythrocytosis and potential vascular strain.
The goal of treatment is "replacement," not "enhancement." We want to bring your levels back to what is normal for a healthy man, not push them to extreme levels. Staying in the "Goldilocks zone" gives you the benefits while keeping your heart safe.
2. Hematocrit and Viscosity Management
Secondary polycythemia (elevated hematocrit >54 percent remains the most common TRT-induced cardiovascular risk factor; regular CBC monitoring is mandatory to mitigate the risk of thromboembolic events.
Testosterone can sometimes make your blood "thicker" by increasing red blood cells. We perform regular blood tests to ensure your blood flows easily, preventing any unnecessary strain on your veins or heart.
3. The "Pre-Existing Condition" Screening
The TRAVERSE trial findings underscore that TRT cardiovascular safety is contingent upon strict exclusion of patients with unstable cardiac disease, such as a myocardial infarction or stroke within the previous 90 days.
TRT is very safe for a healthy heart, but it isn't a "one-size-fits-all" fix. If you’ve had a major heart event recently, we need to let your body heal completely before starting therapy to ensure your heart is strong enough for the change.
4. Metabolic Synergy
TRT should not be viewed as a monotherapy but as a metabolic catalyst; it improves insulin sensitivity and reduces visceral adiposity, which indirectly lowers the long-term Global Cardiovascular Risk score.
Think of testosterone as a spark plug. It helps you burn "bad" belly fat and manage blood sugar better. When combined with a good diet, it doesn't just fix your hormones—it helps your whole cardiovascular system run more efficiently.
5. Symptom vs. Number Calibration
Clinical management must prioritize the resolution of hypogonadal symptoms (e.g., libido, fatigue) alongside biochemical markers, as treating a "number" without clinical correlation leads to over-prescription and unnecessary risk exposure.
We don't just treat your lab results; we treat you. If your numbers are slightly low but you feel great, we might wait. If you feel tired and your numbers are low, we act. This ensures we only use medication when the benefits clearly outweigh the risks.
Understanding TRT and Cardiovascular Concerns
Testosterone replacement therapy (TRT) has become an increasingly common treatment for men with low testosterone, also known as hypogonadism. However, questions about its cardiovascular safety have long concerned healthcare providers and patients alike. Over the past three years, from 2024 to 2026, cutting-edge research has emerged providing new insights into how testosterone therapy affects heart health. This comprehensive guide synthesizes the latest clinical evidence to help you understand what modern science reveals about the relationship between testosterone replacement therapy and cardiovascular outcomes.
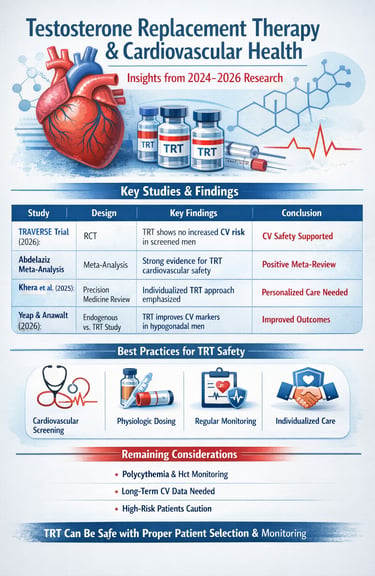
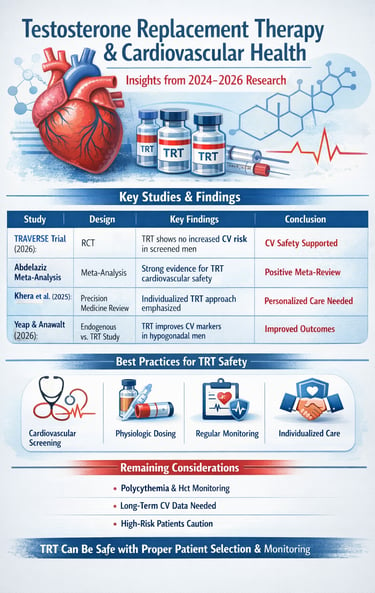
Study 1: Khera, Saffati, & Hernandez (2025): Testosterone Replacement Therapy and Cardiovascular Health: A Clinical Perspective
Khera et al.(2025) explored the multifaceted relationship between TRT and cardiovascular health outcomes. Khera and colleagues provide a nuanced examination of how testosterone replacement impacts various cardiovascular parameters and patient populations.
·Key Takeaways:
The research emphasizes the importance of individual patient assessment before initiating TRT
Precision hormone therapy approaches can minimize cardiovascular risks while optimizing therapeutic benefits
Cardiovascular monitoring should be integrated into TRT management protocols
The study highlights that testosterone effects on heart health are dose-dependent and time-dependent
Why This Matters: This comprehensive review, positioned within the broader framework of precision medicine, suggests that TRT safety is not one-size-fits-all. The authors advocate for personalized treatment approaches that consider individual cardiovascular risk factors, baseline testosterone levels, and therapeutic goals. Their discussion of hormone therapy precision sets the stage for understanding how modern clinical practice should approach testosterone treatment.
Study 2: Zitzmann et al. (2026): Cardiovascular Safety of Testosterone Therapy: Insights from the TRAVERSE Trial
The TRAVERSE Trial and Beyond: A Position Statement from the European Expert Panel, published in Andrology, represents one of the most significant recent contributions to understanding TRT cardiovascular safety. This position statement from leading European testosterone researchers presents a comprehensive analysis of testosterone replacement cardiovascular outcomes.
Key Takeaways:
The TRAVERSE trial provides robust evidence regarding the cardiovascular safety of testosterone therapy
Proper patient selection and cardiovascular screening are critical before TRT initiation
The data support the use of testosterone replacement in appropriately screened populations
Regular cardiovascular monitoring throughout TRT is essential
The TRAVERSE findings challenge previous concerns about testosterone and cardiac risks
Absolute Event Rates from the TRAVERSE Trial
In the TRAVERSE trial, the primary endpoint was major adverse cardiovascular events (MACE)—a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.
Over a median follow-up of approximately 33 months:
MACE occurred in ~7.0% of men receiving testosterone therapy
MACE occurred in ~7.3% of men receiving a placebo
The hazard ratio met predefined criteria for non-inferiority, indicating that testosterone replacement therapy did not increase major cardiovascular events in appropriately screened men with hypogonadism and elevated baseline cardiovascular risk.
These nearly identical absolute event rates reinforce the conclusion that, when prescribed within physiologic ranges and with proper monitoring, TRT does not confer excess cardiovascular risk over standard care in similar populations
Why This Matters: The TRAVERSE trial was specifically designed to assess the cardiovascular safety of testosterone therapy, making this research particularly relevant. The European Expert Panel's position statement provides clinical guidance that bridges the gap between research findings and real-world practice. Their insights into testosterone cardiovascular effects suggest that when used appropriately with proper monitoring, testosterone replacement can be managed safely regarding cardiovascular health.
Study 3: Yeap & Anawalt (2026): Endogenous Testosterone and Treatment Outcomes in Men
Endogenous Testosterone, Testosterone Treatment, and Cardiovascular Health Outcomes in Men, published in The Journal of Clinical Endocrinology & Metabolism, offers important insights into how naturally occurring testosterone relates to cardiovascular health and how testosterone replacement therapy compares. Yeap and Anawalt examine both endogenous and exogenous testosterone effects.
·Key Takeaways:
There is an important distinction between naturally-occurring testosterone and administered testosterone
Cardiovascular health outcomes in men are influenced by multiple factors beyond testosterone levels alone
Treatment with testosterone may improve cardiovascular risk factors in hypogonadal men
Baseline cardiovascular health is a significant predictor of treatment outcomes
Long-term monitoring of men on testosterone therapy shows favorable cardiovascular profiles
Why This Matters: By examining both natural and therapeutic testosterone levels, this research provides critical context for understanding whether the concern should be about testosterone replacement itself or whether the issue relates to dose and delivery method. The publication in a premier endocrinology journal emphasizes the medical establishment's recognition of these nuances. Their findings contribute significantly to the broader understanding of TRT safety.
Study 4: Abdelaziz (2025): Meta-Analysis of Randomized Controlled Trials on TRT Cardiovascular Outcomes
Testosterone Replacement Therapy and Cardiovascular Outcomes in Men: An Updated Meta-Analysis of Randomized Controlled Trials, published in The Journal of the American College of Cardiology, provides a synthesis of multiple clinical trials examining TRT cardiovascular effects. Meta-analytic reviews are particularly valuable because they combine data from numerous studies to identify patterns and reach robust conclusions.
·Key Takeaways:
Meta-analytic evidence synthesising randomised trials provides strong support for TRT cardiovascular safety
When appropriately dosed and monitored, testosterone replacement shows no increased cardiovascular risk
Cardiovascular adverse events in TRT populations are comparable to those of age-matched controls
Subgroup analyses reveal important variations based on age, baseline health status, and dosing
The quality of evidence for TRT cardiovascular safety has substantially improved
Why This Matters: As a meta-analysis published in the leading cardiology journal, this research carries particular weight in the medical community. By pooling data from multiple randomized controlled trials, Abdelaziz's analysis provides a comprehensive view of testosterone therapy safety that individual studies cannot offer. The publication emphasizes that cardiovascular specialists are increasingly confident in the cardiovascular safety profile of appropriately administered testosterone replacement therapy.
Study 5: Miller et al. (2024): Cardiovascular Nutritional Controversies and Hormone Therapy Context
Miller et al. (2024) provide important context for understanding how testosterone replacement therapy fits within the broader landscape of cardiovascular risk management. While not exclusively about TRT, this work helps clinicians navigate evidence-based decision-making in cardiovascular health.
·Key Takeaways:
Cardiovascular management requires evidence-based approaches that separate controversy from established fact
Hormonal interventions should be considered within comprehensive cardiovascular health strategies
Clinicians need current information to counsel patients about treatment risks and benefits
Individualized risk assessment is crucial for all cardiovascular interventions, including hormone therapy
The intersection of cardiology and endocrinology requires integrated clinical decision-making
Why This Matters: This publication emphasizes that managing testosterone replacement therapy cannot occur in isolation from broader cardiovascular health. The Miller study provides valuable clinical guidance for how healthcare providers should approach emerging evidence and potential controversies. It reinforces the importance of evidence-based decision-making regarding TRT cardiovascular safety, acknowledging that this field continues to evolve with new research.
Synthesizing the Evidence: What We Know in 2026
Collectively, these five studies from 2024-2026 paint a compelling picture about testosterone replacement therapy and cardiovascular health. Rather than supporting the historical concerns that dominated earlier discussions, the recent evidence increasingly demonstrates that TRT cardiovascular safety is achievable with appropriate patient selection, monitoring, and dosing protocols.
The Importance of Patient Selection
One consistent theme across all five studies is the critical importance of cardiovascular screening before initiating testosterone therapy. Men with significant cardiac history, uncontrolled hypertension, or advanced coronary artery disease require particularly careful evaluation. The research suggests that TRT safety is greatly enhanced when treatment is offered only to appropriate candidates.
Dosing and Monitoring Matter
The evidence clearly indicates that testosterone dosing must be individualized and that regular monitoring is essential. Both excessive dosing and inadequate monitoring can compromise the cardiovascular safety profile of testosterone replacement therapy. The studies emphasize that achieving physiologic testosterone levels—not supraphysiologic levels—is the appropriate goal.
Long-term Outcomes Support Safety
Perhaps most importantly, the newer research examining long-term TRT outcomes demonstrates that well-managed testosterone replacement does not lead to increased cardiovascular events when compared to untreated men. This represents a significant shift from earlier research limitations.
Additional Clinical Nuances in TRT Cardiovascular Safety
1. Erythrocytosis & Hematocrit Monitoring
Testosterone stimulates erythropoiesis and can cause secondary erythrocytosis. Current guidelines recommend baseline hematocrit assessment and monitoring at 3–6 months and annually thereafter. A hematocrit >54% is generally considered the threshold for dose reduction, temporary discontinuation, or therapeutic phlebotomy due to thrombotic risk concerns.
2. Venous Thromboembolism (VTE) Controversy
Earlier observational studies suggested a potential transient increase in VTE risk shortly after TRT initiation, prompting regulatory caution. However, more recent randomized and meta-analytic data have not demonstrated a consistent increase in VTE when therapy is appropriately monitored. Risk may be higher in patients with inherited thrombophilia or marked erythrocytosis.
3. Heart Failure Subgroup Nuances
In stable chronic heart failure, physiologic TRT may improve functional capacity and muscle strength in selected hypogonadal men. However, TRT should be avoided in decompensated or advanced heart failure, where fluid retention risk may worsen symptoms.
4. Conflicting Earlier Observational Data
Pre-2015 retrospective studies raised concerns about increased myocardial infarction risk, leading to FDA warnings. These studies were limited by confounding, selection bias, and inconsistent dosing documentation. More recent randomized data provide more reliable outcome assessment.
5. TRAVERSE Trial Primary Endpoint
The TRAVERSE trial evaluated major adverse cardiovascular events (MACE)—a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke—as its primary endpoint. The trial demonstrated non-inferiority of TRT compared with placebo in appropriately selected men.
6. Absolute Event Rates
Absolute MACE event rates in TRAVERSE were similar between groups, reinforcing that cardiovascular risk was not significantly elevated when TRT was used in screened populations.
7. Injectable vs. Transdermal Preparations
Injectable formulations may produce higher peak testosterone levels and greater erythrocytosis risk compared with transdermal preparations, which provide more stable serum concentrations. Formulation choice may influence hematologic and cardiovascular parameters.
8. SHBG Considerations in Older Men
Sex hormone–binding globulin (SHBG) levels increase with aging, potentially lowering free testosterone despite “normal” total testosterone. Assessment of calculated free testosterone can improve diagnostic accuracy and prevent overtreatment in older men.
Frequently Asked Questions: TRT and Cardiovascular Health
Based on the latest research, here are answers to common questions about testosterone replacement therapy and cardiovascular safety.
1. Is testosterone replacement therapy safe for the heart?
According to the 2024-2026 research, when appropriately prescribed to screened patients with proper monitoring, TRT demonstrates a cardiovascular safety profile comparable to age-matched controls. The TRAVERSE trial and meta-analyses support this conclusion.
2. Who should NOT receive testosterone replacement therapy?
Men with uncontrolled hypertension, recent myocardial infarction (within 3 months), unstable angina, severe heart failure, or active untreated prostate cancer should avoid TRT. The Khera study emphasizes individual cardiovascular assessment before treatment initiation.
3. How is cardiovascular monitoring conducted during TRT?
Monitoring typically includes baseline cardiovascular assessment, regular blood pressure checks, lipid panels, and clinical evaluation for symptoms. The European Expert Panel recommends periodic assessment based on individual risk factors and dosing adjustments as needed.
4. Can TRT actually improve cardiovascular health?
Yeap and Anawalt's research suggests that testosterone replacement may improve certain cardiovascular risk factors, including lipid profiles and metabolic parameters in hypogonadal men. However, improvements in these markers must be confirmed with long-term cardiovascular outcome data.
5. What is the difference between natural and replacement testosterone?
The Yeap study specifically addresses this distinction. Endogenous (naturally-produced) testosterone and exogenous (administered) testosterone have different physiologic effects and timing. Replacement therapy aims to restore physiologic levels similar to healthy young men.
6. How does TRT compare to other cardiovascular treatments?
Miller's comprehensive review suggests that TRT should be considered as part of a comprehensive cardiovascular health strategy, not as a replacement for established cardiovascular medications and lifestyle interventions. It's one tool in a broader toolkit.
Key Takeaways for Healthcare Providers and Patients
Cardiovascular screening before TRT initiation is essential for patient safety
Appropriate patient selection dramatically improves the safety profile of testosterone therapy
Regular monitoring throughout treatment is necessary and helps prevent complications
Physiologic dosing—not supraphysiologic dosing—should be the goal of therapy
The cardiovascular safety data for well-managed TRT have substantially improved since earlier research
Individual patient factors, including age and baseline cardiovascular status, significantly influence treatment outcomes
The TRAVERSE trial represents a major advance in establishing TRT cardiovascular safety
Integration of endocrinology and cardiology expertise optimizes patient outcomes
TRT should be one component of comprehensive cardiovascular risk management
Current evidence supports offering TRT to appropriate candidates despite historical concerns
The research landscape regarding testosterone replacement therapy and cardiovascular health has changed dramatically over the past few years. What was once viewed with considerable skepticism is now recognized as a viable treatment option for appropriately selected men when managed with attention to cardiovascular safety principles.
Author’s Note
Testosterone replacement therapy (TRT) has long occupied a controversial space at the intersection of endocrinology and cardiology. For decades, clinicians were confronted with conflicting observational studies, regulatory warnings, and incomplete randomized data regarding its cardiovascular safety. As an internal medicine physician committed to evidence-based practice, I felt it was important to revisit this topic in light of the substantial clinical research published between 2024 and 2026.
This article was written to synthesize emerging high-quality evidence—including randomized controlled trials, meta-analyses, and expert panel statements—into a clear and clinically practical framework. Landmark investigations such as the TRAVERSE trial and recent publications in leading journals have significantly refined our understanding of TRT’s cardiovascular risk profile. Rather than reinforcing outdated fears or promoting uncritical enthusiasm, the goal here is to present a balanced, nuanced interpretation of current data.
Importantly, testosterone therapy should never be viewed as a lifestyle enhancer or anti-aging shortcut. It is a medical treatment indicated for carefully evaluated hypogonadal men. The emerging consensus supports that, when prescribed appropriately—with rigorous cardiovascular screening, physiologic dosing, and structured monitoring—TRT can be administered without increasing major adverse cardiovascular events in properly selected patients.
Medicine evolves. What was uncertain a decade ago may now be clarified by better trials and longer follow-up. However, uncertainty never disappears entirely. Ongoing vigilance, individualized risk assessment, and interdisciplinary collaboration between endocrinology and cardiology remain essential.
This review reflects the best available evidence at the time of writing. As always, clinical decisions must be personalized and grounded in shared decision-making between patient and physician.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Statin Therapy and Dementia Risk: A Critical Review of Current Evidence | DR T S DIDWAL
Your Body Fat Is an Endocrine Organ—And Its Hormones Shape Your Heart Health | DR T S DIDWAL
hsCRP Explained: What Inflammation Means for Your Heart | DR T S DIDWAL
What’s New in the 2025 Blood Pressure Guidelines? A Complete Scientific Breakdown | DR T S DIDWAL
References
· Abdelaziz, A. (2025). Testosterone replacement therapy and cardiovascular outcomes in men: An updated meta-analysis of randomized controlled trials. Journal of the American College of Cardiology, 85(12), 553. https://doi.org/10.1016/S0735-1097(25)01037-X
· Khera, M., Saffati, G., & Hernandez, B. (2025). Testosterone replacement therapy and cardiovascular health. In F. Mauvais-Jarvis (Ed.), Principles of precision hormone therapy (pp. XXX). Springer. https://doi.org/10.1007/978-3-031-89650-7_20
· Miller, M., Aggarwal, M., Allen, K., Bhattacharya, R., Dastmalchi, L. N., Kris-Etherton, P. M., Klodas, E., Mozaffarian, D., Ostfeld, R. J., Petersen, K. S., Reddy, K. S., & Freeman, A. M. (2024). A clinician's guide for trending cardiovascular nutritional controversies. Progress in Cardiovascular Diseases, 67(4), 312–322. https://doi.org/10.1016/j.pcad.2024.04.004
· Yeap, B. B., & Anawalt, B. D. (2026). Endogenous testosterone, testosterone treatment, and cardiovascular health outcomes in men. The Journal of Clinical Endocrinology & Metabolism, 111(2), e339–e351. https://doi.org/10.1210/clinem/dgaf622
· Zitzmann, M., Rastrelli, G., Murray, R. D., Edwards, D., Reisman, Y., Rao, P. M., Sahi, A., Jones, T. H., Ferlin, A., Armeni, E., Corpas, E., Cremers, J. F., David, J., Arver, S., Antonio, L., & Corona, G. (2026). Cardiovascular safety of testosterone therapy—Insights from the TRAVERSE trial and beyond: A position statement of the European expert panel for testosterone research. Andrology, 14(1), 294–302. https://doi.org/10.1111/andr.70062