How Exercise Rewires Metabolism: Molecular Control of Lipolysis and Lipid Metabolism
New 2025 research reveals that exercise does more than burn calories—it rewires your molecular blueprints. Discover how perilipin proteins act as gatekeepers for fat loss and how to optimize your workouts for disease prevention and metabolic health
EXERCISEMETABOLISM
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/5/202613 min read

For decades, fat loss has been framed as a simple arithmetic problem—burn more calories than you consume, and adipose tissue will obediently melt away. Yet this model collapses under closer biological scrutiny. Two individuals can perform identical exercise routines, consume similar diets, and display radically different metabolic outcomes. The reason is now becoming unmistakably clear: exercise does not merely expend energy—it rewires lipid metabolism at the molecular level.
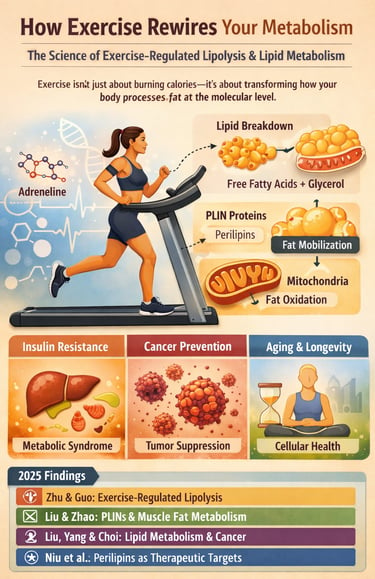
At the center of this transformation lies exercise-regulated lipolysis, a tightly controlled biological process through which physical activity signals fat cells and skeletal muscle to mobilize, transport, and oxidize stored lipids (Zhu & Guo, 2025). This process is not passive. It requires precise coordination between hormones, intracellular signaling cascades, mitochondrial function, and specialized lipid-handling proteins known as perilipins (PLINs). When these systems function optimally, exercise converts stored fat into a readily available energy substrate. When they malfunction, lipid accumulation persists despite physical activity, driving insulin resistance, metabolic syndrome, and chronic disease.
Recent landmark studies published in 2025 have fundamentally reshaped our understanding of how exercise controls lipid metabolism. These investigations reveal that exercise remodels lipid droplet–mitochondria communication in skeletal muscle (Liu & Zhao, 2025), selectively activates perilipins to regulate fat mobilization (Niu et al., 2025), and alters systemic lipid availability in ways that directly influence cancer risk and progression Liu,et al.(2025). Together, this body of evidence establishes exercise as a multi-target metabolic therapy, capable of reshaping endocrine signaling, cellular energy flux, and disease susceptibility across the lifespan.
Understanding these mechanisms is no longer optional. It is foundational to modern approaches to obesity, type 2 diabetes, cancer prevention, and healthy aging.oxidise
Clinical pearls
1. The "Gatekeeper" Protein (PLINs)
Exercise induces the phosphorylation and activation of perilipins (specifically PLIN1-5), which serve as the rate-limiting gatekeepers of the lipid droplet. This molecular remodeling is essential for the translocation of hormone-sensitive lipase (HSL) and the subsequent mobilization of triacylglycerols.
Think of fat in your cells as being locked in a vault. Exercise is the key that turns the lock. Without physical activity, the "gatekeeper" proteins (perilipins) keep the vault shut, even if you are dieting. Exercise "greases the hinges" so your body can actually use that fat for fuel.
2. Metabolic Flexibility & Mitochondrial Tethering
Chronic exercise facilitates organelle "cross-talk," specifically increasing the physical tethering between lipid droplets and mitochondria. This structural adaptation enhances fatty acid oxidation capacity and restores metabolic flexibility, allowing the substrate shift between glucose and lipids.
Exercise doesn't just "burn" fat; it builds a bridge. It physically moves your fat stores closer to your cellular "power plants" (mitochondria). This makes your body an efficient hybrid engine that can easily switch between burning sugar and burning fat, depending on what you're doing.
3. The "Lipotoxic" Traffic Jam
Skeletal muscle insulin resistance is often driven by the accumulation of intramyocellular lipids (IMCLs) due to PLIN dysfunction. Exercise-regulated lipolysis prevents the accumulation of reactive lipid species (like diacylglycerols) that interfere with the insulin signaling pathway.
When you don't move enough, fat gets "stuck" inside your muscles like a traffic jam. This "jam" blocks your insulin from working properly, which can lead to Type 2 Diabetes. Exercise clears the road, letting the fat flow out and helping your insulin work like it’s supposed to.
4. Cancer as a Metabolic Competitor
Exercise modulates the endocrine lipid landscape, reducing the systemic availability of free fatty acids that fuel the rapid proliferation of neoplastic cells. By altering lipid metabolism, exercise creates a "metabolically hostile" microenvironment for tumor progression.
Cancer cells are "energy hungry" and love to eat the extra fat circulating in your blood. When you exercise, you are essentially "starving" potential cancer cells by cleaning up that extra fat and using it yourself. You’re making your body a place where cancer finds it very hard to grow.
5. The Precision Medicine Paradigm
Inter-individual variability in exercise response is partially attributed to genetic polymorphisms in PLIN expression. Patients labeled as "non-responders" may require higher intensity or specific modalities to achieve the threshold of PLIN activation necessary for metabolic improvement.
Everyone’s DNA is different. If you feel like you’re exercising but not seeing "metabolic results," it might just be that your "fat-burning switches" are a little harder to flip. You aren't a "failure"—you might just need a more specific "prescription," like lifting heavier weights or trying high-intensity intervals, to get your system moving.
Understanding the Molecular Magic Behind Fat Burning and Metabolic Health
Why Exercise-Regulated Lipolysis Matters
If you've ever wondered why consistent exercise doesn't just burn calories but actually transforms how your body processes fat, you're asking one of the most important questions in modern health science. The answer lies in a fascinating biological process called exercise-regulated lipolysis—the way physical activity triggers your body to break down and utilize fat stores at the molecular level. This isn't just about fitting into your favorite jeans; it's about preventing chronic diseases, optimizing metabolic health, and building cellular resilience that lasts for decades.
Recent groundbreaking research from 2025 reveals exactly how exercise orchestrates this molecular symphony. Four landmark studies have identified the specific proteins, mechanisms, and pathways that make physical activity one of the most powerful therapeutic interventions ever discovered. Whether you're managing type 2 diabetes, fighting metabolic syndrome, preventing cancer, or simply optimizing your health, understanding lipid metabolism regulation through exercise is essential. Let's dive into what the latest science tells us.
Study 1: Exercise-Regulated Lipolysis—The Comprehensive Overview
The foundational work by Zhu & Guo (2025) provides a sweeping examination of exercise-regulated lipolysis and its role in both health optimization and disease prevention. This comprehensive review synthesizes current knowledge about how physical activity triggers fat breakdown, examining the molecular mechanisms that distinguish healthy lipolysis from pathological lipid accumulation.
Key Findings
Exercise activates exercise-induced lipolysis through multiple coordinated signaling pathways that increase fat oxidation and energy expenditure
The process involves metabolic remodeling that improves the body's ability to shift between carbohydrate and fat utilization, critical for metabolic flexibility
Exercise-regulated lipid metabolism protects against obesity, type 2 diabetes, cardiovascular disease, and metabolic syndrome when performed consistently
The therapeutic potential extends to ageing-related metabolic decline and chronic disease prevention across the lifespan
Understanding exercise-regulated lipolysis mechanisms provides the foundation for optimizing exercise prescriptions. Rather than viewing exercise as simply burning calories, Zhu and Guo's work demonstrates that physical activity is a sophisticated biological intervention that rewires how your cells process energy. This paradigm shift explains why two people can do identical workouts but achieve different metabolic outcomes—individual differences in these molecular pathways matter tremendously. The implications for personalized medicine are profound: by understanding which lipid metabolism pathways are most active in your body, clinicians could eventually optimize exercise prescriptions based on your unique molecular profile.
Study 2: PLINs-Mediated Organelle Interactions and Skeletal Muscle Lipid Metabolism
Now let's zoom in on a specific molecular player: perilipins (PLINs). Liu & Zhao( 2025) focused on how these critical proteins orchestrate communication between cellular organelles to regulate skeletal muscle lipid metabolism. This study unveils the intricate dance between lipid droplets, mitochondria, and other cellular structures that determines whether muscle cells burn fat efficiently or allow lipid accumulation.
Key Findings
Perilipins function as lipid droplet coat proteins that regulate how readily fat is released from storage for energy production
Exercise stimulates PLIN-mediated signaling that enhances communication between lipid droplets and mitochondria, allowing muscle cells to access stored fat for oxidation
Abnormal PLIN expression contributes to skeletal muscle lipid accumulation, a hallmark of insulin resistance and metabolic dysfunction
Exercise-induced improvements in skeletal muscle lipid metabolism disorders depend critically on proper PLIN regulation and organelle communication
The Liu and Zhao study reveals a previously underappreciated layer of metabolic control: the subcellular choreography of lipid handling. Most people think of fat metabolism as a simple input-output equation, but this research shows it's far more sophisticated. PLINs essentially act as gatekeepers deciding how much fat can flow from storage sites to mitochondria for burning. When you exercise, these proteins are remodeled—phosphorylated and activated—to increase fat availability. This explains why sedentary individuals often develop lipid droplet accumulation in muscle despite not eating excessively. Their PLINs aren't properly functioning, creating a traffic jam of energy-rich fat that can't reach the mitochondrial furnace. This discovery opens possibilities for therapeutic interventions targeting PLIN dysfunction in conditions like type 2 diabetes and metabolic syndrome.
Study 3: Modulation of Lipid Metabolism by Exercise in Cancer Endocrinology
Perhaps the most exciting frontier in exercise-regulated lipid metabolism research is its role in cancer prevention and treatment. Liu et al. (2025) explored how exercise modulates lipid metabolism to create an anti-cancer metabolic environment. This research reveals that the way your body handles fat directly influences cancer risk and therapeutic response—a connection most people never consider.
Key Findings
Cancer cells exhibit dramatically altered lipid metabolism, often depending on increased lipid synthesis for rapid proliferation
Exercise reduces circulating lipids and shifts the metabolic environment away from the lipid-rich state that tumours prefer, serving as cancer prevention through metabolic modulation
Physical activity enhances the body's immune response while simultaneously creating an unfavorable endocrine lipid environment that suppresses tumor growth
The combination of exercise-mediated lipid metabolism changes and improved insulin sensitivity creates multiple overlapping protective effects against cancer initiation and progression
This research elevates exercise from a general health habit to a precision cancer prevention tool. Cancer cells are metabolically hungry, preferring to feed on readily available lipids rather than engaging in energy-expensive metabolic processes. By exercising regularly and maintaining healthy lipid profiles, you're literally removing fuel from potential tumor cells. The study demonstrates that exercise acts as a cancer metabolism therapeutic by rewriting your body's endocrine lipid landscape. This finding is particularly exciting for cancer survivors and those at high genetic risk—exercise becomes not just supportive care but a core therapeutic strategy. The implications extend to cancer treatment resistance; understanding how lipid metabolism modulation affects tumor biology could enhance the effectiveness of existing chemotherapy and immunotherapy approaches.
Study 4: Perilipins as Key Targets for Exercise-Mediated Lipid Metabolism Improvement
Niu et al.(2025) brought everything together by specifically establishing perilipins as therapeutic targets for exercise-mediated improvement of abnormal lipid metabolism. While the Liu-Zhao study examined PLIN function broadly, this research goes deeper by characterizing which specific PLIN subtypes are most important for different metabolic conditions and how various exercise modalities activate them.
Key Findings
Different perilipins (PLIN1-5) play specialized roles; for example, PLIN1 primarily regulates adipose tissue lipid storage while PLIN2-5 control lipid handling in muscle and other tissues
Exercise selectively upregulates and activates specific perilipins depending on exercise type, intensity, and duration—creating exercise-specific lipid metabolic responses
Individuals with perilipin genetic variations or reduced PLIN expression show blunted metabolic responses to exercise, explaining exercise non-responders in clinical studies
Optimizing exercise prescription to target perilipin regulation could enable precision exercise medicine for metabolic disorders and provide therapeutic alternatives to pharmacological interventions
The Niu study is where molecular biology meets clinical reality. It answers a question that's puzzled exercise scientists for decades: why does exercise produce wildly different metabolic results in different people? The answer is partly biological—some people's perilipin genes simply respond more robustly to exercise signals. This doesn't mean non-responders are stuck; rather, it means they may need exercise prescription personalization. More frequent training, different exercise modalities (aerobic versus resistance), or higher intensities might unlock greater PLIN activation in these individuals. The research also suggests that future therapeutic interventions could directly target perilipin expression or activation—essentially pharmacologically mimicking what exercise does naturally. For people with diabetes, metabolic syndrome, or lipid disorders, this represents a pathway toward perilipin-targeted therapy that could supplement or eventually enhance exercise-based interventions.
The Integrated Model: How These Studies Work Together
While each study contributes unique insights, their real power emerges when viewed as an integrated model of exercise-regulated lipid metabolism. Imagine your body as a sophisticated energy management system:
Zhu and Guo provide the architectural blueprint—they map the major signaling highways that exercise activates. Liu and Zhao zoom into the cellular garage where fat is actually stored and mobilized; they show us how perilipins act as forklift operators moving fat from storage droplets to the mitochondrial power plants. Liu, Yang, and Choi reveal that this entire system has profound implications for disease prevention, particularly cancer resistance. Finally, Niu and colleagues give us the detailed instruction manual for optimizing each component—showing which perilipins to target and how different exercise approaches activate them.
Together, these studies reveal that exercise doesn't simply burn calories through muscular effort. Instead, it orchestrates a comprehensive metabolic rewiring through lipid metabolism regulation that influences everything from body composition to cancer risk to insulin sensitivity
The Myokine Connection: Muscle as an Endocrine Organ
While perilipins manage fat inside the muscle, myokines (a subset of "exerkines") are the chemical messengers that allow muscles to communicate with the rest of the body. When your muscles contract, they act as an endocrine gland, secreting hundreds of these signaling molecules into the bloodstream.
Irisin: The "Browning" Agent: One of the most famous myokines, Irisin, travels to white adipose tissue (the "storage" fat) and signals it to transform into beige fat. Beige fat is rich in mitochondria and burns energy to produce heat, rather than just sitting in storage.
IL-6 (Interleukin-6): The Lipolysis Trigger: During exercise, muscle-derived IL-6 spikes, acting directly on distant fat depots to accelerate the breakdown of fats (lipolysis) and increase the delivery of fatty acids to the working muscles.
The Systemic Effect: These exerkines explain why leg exercises can help reduce fat systemically—they create a "metabolic ripple effect" that improves insulin sensitivity in the liver and reduces inflammation in the brain.
Clinical Applications: What This Means for You
Type 2 Diabetes and Metabolic Syndrome
These studies collectively suggest that exercise-induced improvements in lipid metabolism are core to diabetes reversal. By activating perilipin-mediated fat mobilization, regular exercise prevents the lipid accumulation in muscle that drives insulin resistance. The implication: if you've been diagnosed with type 2 diabetes, optimizing your exercise-regulated lipolysis through consistent physical activity should be a primary therapeutic goal, potentially as important as medication adjustment.
Weight Management and Metabolic Health
Rather than viewing weight loss as purely caloric restriction, these findings reframe it as optimizing metabolic flexibility and exercise-regulated fat oxidation. Two people eating identical calories will lose weight differently based on their lipid metabolism capacity. Exercise that effectively activates lipid mobilization pathways will produce superior metabolic outcomes.
Cancer Prevention and Survivorship
For cancer prevention, the research suggests that maintaining lean body composition through exercise-mediated lipid metabolism is directly protective. Cancer cells preferentially utilize excess circulating lipids; by exercising regularly and maintaining healthy lipid profiles, you create a metabolically hostile environment for tumor development. For cancer survivors, therapeutic exercise targeting lipid metabolism optimization should be integrated into survivorship care plans.
Aging and Longevity
Age-related metabolic decline involves progressive loss of lipid metabolism flexibility and reduced capacity for exercise-induced fat oxidation. These studies suggest that maintaining active perilipins and healthy skeletal muscle lipid metabolism through lifelong exercise is a foundational anti-aging strategy.
Evidence-Based Exercise Recommendations for Optimizing Lipid Metabolism
Aerobic Exercise
Sustained aerobic exercise preferentially activates lipolytic pathways and maximizes fat oxidation. The research supports 150 minutes weekly of moderate-intensity aerobic activity as effective for stimulating exercise-regulated fat metabolism.
Resistance Training
Strength training builds muscle tissue that serves as a major site of lipid-responsive metabolism. Increased skeletal muscle mass directly improves whole-body lipid metabolism efficiency and creates metabolic durability for lifelong health.
High-Intensity Interval Training (HIIT)
HIIT creates acute metabolic stress that strongly activates perilipins and lipolytic signaling. The metabolic afterburn effect means elevated fat oxidation persists hours after exercise. Even brief HIIT sessions (15-20 minutes) can powerfully stimulate exercise-induced fat mobilization.
Frequently Asked Questions
What exactly is exercise-regulated lipolysis?
Exercise-regulated lipolysis is the biological process by which physical activity signals your body to break down stored fat (triglycerides) into usable energy molecules (free fatty acids and glycerol). It's regulated by hormones like adrenaline and controlled at the cellular level by proteins like perilipins that manage lipid droplet mobilization.
Why are perilipins so important?
Perilipins are protein coats that surround fat droplets inside cells. They function as gatekeepers controlling whether fat can be released for energy production. Exercise activates perilipins through phosphorylation, essentially 'opening the gates' to allow fat mobilization. Without healthy perilipins, fat remains trapped in cells even during exercise.
If I have abnormal lipid metabolism, can exercise fix it?
In most cases, yes. These studies demonstrate that exercise improves abnormal lipid metabolism by restoring healthy PLIN function, enhancing mitochondrial lipid oxidation, and improving metabolic flexibility. However, some individuals have genetic variations affecting perilipin function (making them exercise non-responders), and these may require personalized exercise prescription or supplemental interventions.
How does exercise-regulated lipolysis prevent cancer?
Cancer cells thrive in a lipid-rich environment where abundant fatty acids fuel rapid proliferation. Exercise reduces circulating lipids, maintains lean body mass, improves insulin sensitivity, and enhances immune function—collectively creating a metabolically hostile environment for tumor development. The mechanism involves both exercise-mediated lipid metabolism changes and systemic metabolic improvements.
How long does it take for exercise to improve lipid metabolism?
Acute effects occur within hours—exercise immediately activates lipolytic pathways and perilipins. Sustained improvements in resting lipid metabolism and perilipin expression typically emerge within 2-4 weeks of consistent training. Significant changes in body composition and metabolic health generally require 8-12 weeks of regular exercise.
Key Takeaways
Exercise-Regulated Lipolysis:
Physical activity activates coordinated molecular signaling pathways that enhance fat mobilization and oxidation, leading to long-term rewiring of metabolic capacity rather than temporary calorie expenditure.Perilipins as Metabolic Gatekeepers:
Perilipin (PLIN) proteins regulate access to stored intracellular fat. Exercise activates these proteins, enabling efficient transfer of fatty acids to mitochondria for oxidation and energy production.Cancer Prevention via Metabolic Modulation:
Exercise lowers circulating lipid availability and improves metabolic signaling, creating an endocrine and cellular environment that is unfavorable for tumor initiation and progression.Precision Exercise Medicine:
Genetic and expression differences in perilipins contribute to variable metabolic responses to exercise. Personalized exercise prescriptions can optimize lipid metabolism and therapeutic outcomes across individuals.
Author’s Note
This article was written to bridge an important gap between molecular exercise biology and everyday clinical practice. For too long, physical activity has been discussed in terms of calories burned or minutes logged, while its profound effects on cellular metabolism have remained underappreciated outside academic circles. The studies cited here—published in 2025—represent a pivotal moment in exercise science, revealing that exercise functions as a targeted regulator of lipid metabolism through mechanisms such as exercise-regulated lipolysis, perilipin activation, and lipid droplet–mitochondrial communication.
As a clinician, I have seen how metabolic diseases like type 2 diabetes, dyslipidemia, and obesity are often treated primarily with pharmacological tools, while exercise is framed as optional lifestyle advice. The emerging science challenges that hierarchy. Physical activity is not merely supportive care; it is a biologically active intervention capable of reshaping insulin sensitivity, lipid handling, inflammatory tone, and even cancer-related metabolic pathways.
This piece is intended for clinicians, researchers, and scientifically curious readers who want to understand why exercise works, not just that it works. While the molecular pathways discussed are complex, the message is simple: consistent movement sends powerful signals to our cells, signals that no pill can fully replicate. Exercise remains one of the few interventions that simultaneously improves metabolic health, resilience to disease, and long-term functional capacity.
I hope this article encourages a more mechanistic, evidence-based appreciation of exercise—and supports its integration as a core therapeutic strategy in modern medicine.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Why Metabolic Flexibility May Be the Missing Link in Longevity and Healthy Aging | DR T S DIDWAL
Your Body Fat Is an Endocrine Organ—And Its Hormones Shape Your Heart Health | DR T S DIDWAL
Movement Snacks: How VILPA Delivers Max Health Benefits in Minutes | DR T S DIDWAL
References
Liu, H., Yang, T., & Choi, S. (2025). Modulation of lipid metabolism by exercise: Exploring its potential as a therapeutic target in cancer endocrinology. Frontiers in Endocrinology, 16, Article 1580559. https://doi.org/10.3389/fendo.2025.1580559
Liu, Z., & Zhao, Y. (2025). PLINs-mediated organelle interactions: A key of exercise-mediated improvement of skeletal muscle lipid metabolism disorders. Frontiers in Endocrinology, 16, Article 1700668. https://doi.org/10.3389/fendo.2025.1700668
Niu, Y. J., Liu, J. J., Zhang, J. H., et al. (2025). Perilipins: Key targets for regulating lipid metabolism and alleviating abnormal lipid metabolism through exercise. Diabetology & Metabolic Syndrome, 17, 392. https://doi.org/10.1186/s13098-025-01965-5
Zhu, J. Y., & Guo, L. (2025). Exercise-regulated lipolysis: Its role and mechanism in health and diseases. Journal of Advanced Research, 75, 291–309. https://doi.org/10.1016/j.jare.2024.11.031