Branched-Chain Amino Acids in Health and Disease: Mitochondrial Dysfunction and the Biology of BCAA Metabolism
Explore how branched-chain amino acids shape metabolism, insulin sensitivity, cardiovascular risk, and cancer—revealing why BCAA metabolism matters more than you think.
NUTRITION
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/8/202612 min read
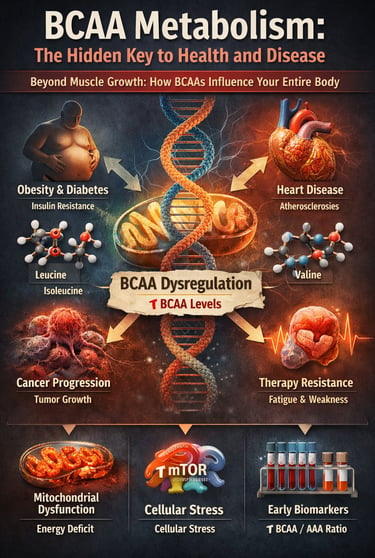
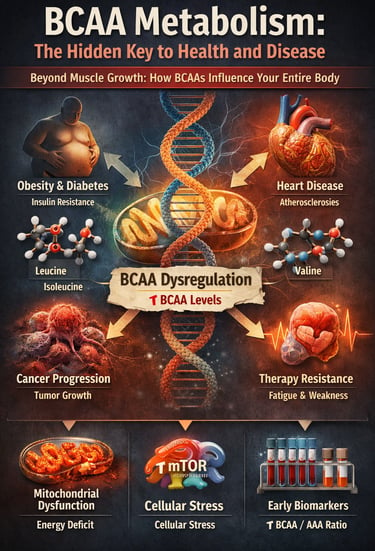
For decades, branched-chain amino acids (BCAAs) have been framed almost exclusively through the lens of muscle growth and athletic performance—a narrow view that now appears profoundly incomplete. Emerging evidence from large-scale metabolomic studies and mechanistic investigations reveals that BCAA metabolism sits at the crossroads of energy regulation, insulin signaling, mitochondrial health, and disease progression. Far from being metabolically neutral nutrients, BCAAs actively shape the trajectory of metabolic health and chronic disease risk (Choi et al., 2024).
Prospective cohort studies consistently demonstrate that elevated circulating BCAA levels predict the future development of obesity, type 2 diabetes, cardiovascular disease, and certain cancers—often years or even decades before clinical diagnosis (Mansoori et al., 2025; Yin et al., 2025). These associations persist after adjustment for total protein intake, physical activity, and adiposity, suggesting that the issue is not BCAA consumption alone, but dysregulated BCAA catabolism and impaired mitochondrial oxidation.
At the cellular level, defective BCAA metabolism leads to accumulation of branched-chain α-ketoacids and downstream metabolites that disrupt mitochondrial function, activate maladaptive mTOR signaling, and promote insulin resistance, endothelial dysfunction, and cellular stress responses (Choi et al., 2024; Zhou et al., 2025). In parallel, clinical studies increasingly show that BCAA/AAA ratios and fasting plasma BCAA concentrations outperform traditional biomarkers in predicting metabolic deterioration (Costa et al., 2025).
These findings force a paradigm shift: BCAAs are no longer passive building blocks but active metabolic signals whose mismanagement accelerates cardiometabolic disease and therapy resistance in cancer. Understanding BCAA metabolism is therefore not optional—it is central to modern preventive and precision medicine.
Clinical pearls
1. The "Metabolic Overflow" Principle
Scientific Perspective: Chronic elevation of circulating BCAAs often reflects a "bottleneck" in the BCKDC enzyme complex—the primary engine for BCAA oxidation. When this engine stalls, BCAAs back up in the blood, signaling mitochondrial distress.
Think of your metabolism like a sink. If the drain (your enzymes) is clogged, the water (BCAAs) overflows. Having high BCAA levels isn't usually about eating too much protein; it’s a sign that your "metabolic drain" needs fixing.
2. Context is King: Muscle vs. Fat
Scientific Perspective: In lean, active individuals, BCAAs are partitioned toward muscle protein synthesis via mTORC1. However, in the context of obesity and high-fat intake, that same mTOR signaling becomes a driver of insulin resistance and endoplasmic reticulum (ER) stress.
BCAAs are "smart" molecules that act differently depending on your body composition. In an athlete, they build muscle. In a sedentary person with a poor diet, they can accidentally tell the body to stop responding to insulin.
3. The Cardiovascular "Silent Signal"
Scientific Perspective: Emerging 2025 data suggest that BCAA metabolites, specifically branched-chain alpha-ketoacids (BCKAs), directly impair endothelial nitric oxide synthase. This reduces the blood vessels' ability to dilate, contributing to hypertension.
Your BCAA levels might be an early warning system for your heart. When these amino acids aren't processed correctly, they produce "toxic exhaust" that can stiffen your arteries long before your blood pressure officially spikes.
4. Exercise as an Enzyme "Primer"
Scientific Perspective: Physical activity is the most potent non-pharmacological upregulator of BCAA catabolism. Both aerobic and resistance training increase the expression of PP2Cm, the phosphatase that activates the BCAA-clearing machinery.
Exercise is the "Drano" for your metabolic sink. Moving your muscles doesn't just burn calories; it physically turns on the enzymes that clear out BCAAs, preventing them from hanging around and causing metabolic mischief.
5. The Cancer "Fuel Hijack"
Scientific Perspective: Many aggressive tumors upregulate LAT1 transporters to "hoover up" leucine from the blood. This isn't just for building blocks; the tumor uses BCAA signaling to bypass normal growth-control checkpoints and resist chemotherapy.
Cancer cells are greedy for BCAAs. They use them as both a high-octane fuel and a communication tool to survive treatment. This makes BCAA management a potential "secret weapon" in future cancer therapies.
6. The BCAA/AAA Balance
Scientific Perspective: The ratio of Branched-Chain Amino Acids to Aromatic Amino Acids (Phenylalanine, Tyrosine, Tryptophan) is a sensitive marker of liver health and systemic metabolic stress. A low ratio often precedes clinical hepatic encephalopathy or severe metabolic syndrome.
Your body tries to keep different types of amino acids in a strict balance. If the ratio between your "branched" and "aromatic" amino acids gets out of whack, it's a major red flag that your liver or muscles are struggling to keep up with your body's demands.
Why BCAAs Matter More Than You Think
When it comes to optimizing your health and preventing chronic diseases, few molecular players are as influential—yet underappreciated—as branched-chain amino acids (BCAAs). These three essential amino acids—leucine, isoleucine, and valine—aren't just building blocks for muscle; they're metabolic maestros orchestrating a complex symphony of cellular processes that directly impact everything from your waistline to your heart health and even your body's ability to fight cancer.
But here's the problem: for decades, we've oversimplified our understanding of BCAA metabolism. Recent groundbreaking research from 2024-2025 reveals that the relationship between BCAAs and human health is far more nuanced—and far more critical—than previously thought.
In this comprehensive guide, we'll explore what the latest science tells us about BCAA metabolic dysfunction, its role in metabolic diseases, cardiovascular complications, and cancer progression. Whether you're a health-conscious individual, a fitness enthusiast, or a healthcare professional, understanding BCAA physiology could be the missing piece in your health puzzle.
What Are BCAAs? Understanding the Basics
Branched-chain amino acids (BCAAs) represent approximately 35-40% of the essential amino acids in your body. Unlike other amino acids that are metabolized primarily in the liver, BCAAs are uniquely processed in muscle and other peripheral tissues, making them extraordinarily important for metabolic regulation.
The three BCAA components are:
Leucine (the metabolic powerhouse)
Isoleucine (the regulator)
Valine (the balancer)
These amino acids participate in numerous metabolic pathways that extend far beyond protein synthesis. They influence energy metabolism, insulin signaling, mitochondrial function, and gene expression—mechanisms that are central to modern chronic disease prevention.
Study 1: Pathophysiological Mechanisms and Therapeutic Interventions
Mansoori and colleagues conducted a comprehensive examination of BCAA metabolism and its direct relationship to metabolic disease development (Mansoori et al., 2025). Published in Obesity Reviews, this work represents one of the most current syntheses of how branched-chain amino acid pathways become dysregulated in obesity and related conditions.
The research identifies several critical mechanisms by which BCAA dyshomeostasis—an imbalance in BCAA metabolism—contributes to metabolic disease:
Mitochondrial Dysfunction: Impaired BCAA catabolism leads to accumulation of branched-chain metabolites, which directly damage mitochondria and reduce energy production efficiency.
Insulin Resistance: Elevated circulating BCAAs and their metabolites interfere with insulin signaling pathways, particularly through mTOR pathway overactivation and increased endoplasmic reticulum stress.
Amino Acid Imbalance: BCAA/AAA ratios (branched-chain to aromatic amino acids) become disrupted, perpetuating metabolic dysfunction in a vicious cycle.
The study emphasizes that therapeutic interventions must target the root cause of BCAA metabolic dysfunction rather than simply restricting amino acid intake. Promising approaches include:
Modulating BCAA-metabolizing enzymes like branched-chain α-ketoacid dehydrogenase (BCKDC)
Using targeted nutritional interventions to restore proper amino acid homeostasis
Implementing lifestyle modifications that enhance mitochondrial biogenesis
Key Takeaway: Understanding the pathophysiology of altered BCAA metabolism opens doors to precision medicine approaches that could reverse metabolic disease progression.
Study 2: The Role of BCAA Metabolism in Metabolic Health and Disease
This 2024 publication in Experimental & Molecular Medicine provides a mechanistic deep-dive into how BCAA metabolic pathways distinguish between health-promoting and disease-promoting states (Choi et al., 2024). The authors argue that BCAA metabolism isn't simply a marker of disease—it's a fundamental driver.
The research distinguishes between metabolically healthy and metabolically unhealthy phenotypes through the lens of BCAA homeostasis:
In metabolically healthy individuals:
BCAA clearance is efficient and context-appropriate
BCAA-derived metabolites (like acetyl-CoA) are properly utilized for energy
Mitochondrial capacity allows for appropriate BCAA oxidation
Insulin sensitivity ensures proper nutrient partitioning
In disease states:
Impaired BCAA oxidation leads to plasma accumulation
Branched-chain metabolite toxicity damages cellular structures
Altered BCAA signaling through mTOR and AMPK disrupts metabolic regulation
Circulating BCAA levels become predictive of metabolic dysfunction progression
The study identifies BCAA metabolism as a "metabolic thermostat"—when working properly, it maintains homeostasis; when dysregulated, it accelerates disease development. This has profound implications for:
Key Takeaway: BCAA metabolic status could serve as an early warning system for metabolic disease, potentially decades before conventional markers show abnormalities.
Study 3: Clinical Practice Applications and Integrative Review
Costa and colleagues present a comprehensive integrative review that bridges the gap between basic BCAA metabolism research and real-world clinical application (Costa et al., 2025). This work synthesizes current evidence on how branched-chain amino acids function across various clinical contexts.
The integrative review identifies several high-impact clinical scenarios where BCAA metabolism becomes therapeutically relevant:
1. Hepatic Disease and Liver Dysfunction
BCAA supplementation demonstrates potential benefits in hepatic encephalopathy
Altered BCAA/AAA ratios predict liver disease severity
Liver-specific BCAA metabolism becomes impaired in cirrhosis and fatty liver disease
2. Renal Disease Management
BCAA metabolism becomes critical in end-stage renal disease
Uremic toxins and impaired BCAA catabolism interact destructively
Amino acid composition in dialysis solutions requires BCAA optimization
3. Post-Surgical Recovery
BCAA administration supports tissue healing and metabolic recovery
Surgical stress and altered BCAA metabolism require nutritional optimization
Immune function depends on appropriate BCAA metabolic status
The review emphasizes that BCAA supplementation isn't universally beneficial—context matters enormously:
Key Takeaway: BCAA metabolic assessment should become a standard part of nutritional evaluation in clinical practice, with interventions tailored to individual BCAA metabolism patterns.
Study 4: BCAA Metabolism and Cardiovascular Disease Risk
One of the most startling recent discoveries concerns the link between BCAA metabolism and cardiovascular disease. Yin and colleagues, in this 2025 American Journal of Cardiovascular Drugs publication, elucidate how abnormal BCAA metabolism directly involves pathogenic mechanisms in heart disease (Yin et al., 2025).
Mechanisms Linking BCAA Metabolism to Cardiovascular Pathology
1. Endothelial Dysfunction
Elevated circulating BCAAs impair endothelial nitric oxide production
BCAA metabolite accumulation increases oxidative stress in blood vessels
BCAA-mediated mTOR activation disrupts endothelial homeostasis
2. Atherosclerosis Acceleration
Impaired BCAA oxidation promotes atherogenic metabolite production
BCAA and atherosclerosis associations are mediated through inflammation
Branched-chain metabolites promote foam cell formation in arterial walls
3. Heart Failure Progression
Cardiac BCAA metabolism becomes severely impaired in heart failure
BCAA accumulation in failing myocardium contributes to fibrosis
Energy crisis in heart failure partly results from dysfunctional BCAA oxidation
4. Arrhythmia Development
Metabolite-induced electrolyte dysbalance from impaired BCAA catabolism
Oxidative stress from defective BCAA metabolism damages cardiac conduction
Therapeutic Intervention Strategies:
Enhancing BCAA catabolic enzyme activity through pharmacological means
Dietary BCAA modulation tailored to individual cardiovascular risk
Metabolite sequestration strategies to prevent toxic accumulation
Exercise-based interventions that improve cardiac BCAA metabolism
Key Takeaway: BCAA metabolic status may be an overlooked cardiovascular risk factor, and optimizing BCAA metabolism could represent a novel prevention strategy for heart disease.
Study 5: BCAA Metabolism in Cancer Biology and Therapy Resistance
Perhaps most intriguingly, Zhou and colleagues reveal in their Frontiers in Pharmacology publication how cancer cells exploit BCAA metabolism to survive and resist treatment (Zhou et al., 2025). This work illuminates why BCAA metabolic dysregulation may be central to cancer biology.
BCAA metabolic reprogramming in cancer cells involves:
1. Enhanced BCAA Uptake
Oncogenic transformation dramatically increases BCAA transporter expression (LAT1, LAT2, 4F2hc)
Tumor-specific BCAA metabolism creates a metabolic dependency
Cancer cell BCAA demand often exceeds that of surrounding normal tissue
2. Rewired BCAA Oxidation
Cancer cells alter BCAA catabolic flux toward specialized metabolite production
Branched-chain metabolites fuel lipid synthesis and cell proliferation
mTOR pathway activation by BCAA metabolites promotes tumor growth
3. Signaling Functions Beyond Energy
BCAA-mediated mTOR signaling suppresses autophagy, allowing therapy resistance
Leucine signaling activates survival pathways independent of energy status
BCAA metabolite-induced transcription reprograms cancer cell identity
The research suggests why many cancers develop therapy resistance:
Chemotherapy selects for cells with enhanced BCAA metabolic capacity
BCAA-dependent mTOR signaling protects cells from therapeutic stress
BCAA metabolic adaptation precedes clinical treatment failure
Key Takeaway: BCAA metabolism is not merely a marker of cancer aggressiveness—it's a driver of therapy resistance, and metabolic intervention targeting BCAA pathways could enhance cancer treatment efficacy.
Synthesizing the Evidence: Common Themes Across Five Breakthrough Studies
While each study addresses distinct conditions, several overarching themes emerge about BCAA metabolic dysfunction:
Theme 1: The Mitochondrial Core Problem
Across metabolic disease, cardiovascular disease, and cancer, the fundamental problem involves impaired BCAA oxidation and mitochondrial dysfunction. Whether from obesity, aging, or malignant transformation, tissues lose the capacity to properly metabolize BCAAs.
Theme 2: Metabolites Matter More Than Amino Acids
The amino acids themselves aren't the only culprits—branched-chain metabolites (like branched-chain α-ketoacids and their downstream products) are often more pathogenic than elevated BCAAs alone.
Theme 3: Context-Dependent Intervention
BCAA supplementation isn't universally beneficial. The same intervention could be therapeutic in one context (hepatic encephalopathy) and harmful in another (insulin-resistant obesity). Personalized BCAA metabolism assessment is essential.
Theme 4: Early Biomarker Potential
Across all conditions, circulating BCAA levels and BCAA/AAA ratios emerge as early predictive markers for disease development—potentially decades before conventional diagnostics detect problems.
Frequently Asked Questions About BCAA Metabolism
Q1: Should Everyone Supplement with BCAAs?
A: Not necessarily. While BCAAs are essential amino acids, supplementation is only beneficial if you have documented BCAA metabolic dysfunction or specific clinical indications. Excessive BCAA supplementation in metabolically healthy individuals may worsen insulin resistance and accelerate metabolic aging. Consult healthcare providers who understand individual BCAA metabolic status before supplementing.
Q2: How Do I Know If I Have BCAA Metabolic Dysfunction?
A: Clinical signs include unexplained weight gain despite moderate caloric intake, insulin resistance markers (high fasting insulin, elevated HbA1c), fatigue despite adequate sleep, impaired exercise recovery, and family history of metabolic or cardiovascular disease. Advanced assessment involves measuring plasma BCAA levels and BCAA/AAA ratios, which increasingly should be part of comprehensive metabolic screening.
Q3: What Diet Optimizes BCAA Metabolism?
A: Research suggests that moderately high protein intake with balanced amino acid composition (not excessive BCAAs) supports healthy BCAA metabolism. Mediterranean-style diets rich in plant proteins, whole grains, and omega-3 fatty acids appear superior to high-BCAA, isolated supplementation approaches. BCAA metabolic capacity is enhanced by regular aerobic and resistance exercise.
Q4: Can BCAA Metabolic Status Predict Disease Risk?
A: Emerging evidence strongly suggests yes. Elevated fasting BCAA levels and elevated BCAA/AAA ratios predict development of type 2 diabetes, metabolic syndrome, cardiovascular disease, and even certain cancers—often 10-20 years before clinical diagnosis. BCAA metabolic assessment should become part of routine preventive medicine.
Q5: Are There Natural Ways to Improve BCAA Metabolism?
A: Absolutely. BCAA metabolic efficiency improves through: regular aerobic exercise (which enhances BCAA oxidation in muscle), resistance training (which increases BCAA-metabolizing capacity), caloric restriction (which prevents BCAA accumulation), quality sleep (essential for mitochondrial BCAA metabolism), and stress reduction (since chronic stress impairs BCAA catabolism).
Q6: Does BCAA Metabolism Change with Age?
A: Yes, significantly. Age-related BCAA metabolic dysfunction represents a key feature of metabolic aging and sarcopenia. Older adults often demonstrate reduced BCAA oxidative capacity and elevated fasting BCAA levels, even without overt metabolic disease. This makes BCAA metabolic support particularly important in aging populations.
Q7: Can Medications Affect BCAA Metabolism?
A: Many commonly used medications affect BCAA metabolism: corticosteroids increase BCAA catabolism, metformin may improve BCAA oxidation, while certain psychiatric medications can impair metabolic BCAA handling. If you're on chronic medications, discuss potential BCAA metabolic effects with your healthcare provider.
Call to Action: Take Control of Your BCAA Metabolism
The science is clear: BCAA metabolism profoundly influences your health trajectory. You have three immediate steps:
Step 1: Assess Your Current Status Discuss BCAA metabolic assessment with your healthcare provider. Request fasting BCAA levels and BCAA/AAA ratios as part of your next comprehensive health evaluation. These simple blood tests provide invaluable insight into your metabolic health.
Step 2: Optimize Your Lifestyle Foundation Don't wait for supplements or medications. Start today with what you control: consistent aerobic and resistance exercise, balanced nutrition emphasizing whole foods and proper amino acid balance, 7-9 hours of quality sleep nightly, and evidence-based stress reduction practices.
Step 3: Develop a Personalized Plan If you have metabolic concerns, cardiovascular risk factors, cancer history, or are struggling with unexplained metabolic dysfunction, schedule a consultation with a healthcare provider versed in metabolic medicine and BCAA metabolism. Personalized BCAA metabolic optimization could literally change your health trajectory.
Your BCAA metabolism is responding to your daily choices right now. Make them count.
Author’s Note
Branched-chain amino acids occupy a unique and often misunderstood position in human metabolism. While they have long been celebrated for their role in muscle protein synthesis and athletic performance, emerging evidence makes it clear that BCAA metabolism extends far beyond the gym, influencing insulin sensitivity, mitochondrial function, cardiovascular health, and even cancer biology. This article was written to challenge reductionist narratives and to present BCAAs as active metabolic signals rather than inert nutritional building blocks.
The intent is not to promote or discourage BCAA supplementation in isolation, but to emphasize context, metabolic capacity, and biological individuality. Many of the adverse associations attributed to BCAAs arise not from intake alone, but from impaired oxidation, mitochondrial dysfunction, and disrupted amino acid balance—processes that often precede overt disease. Understanding these mechanisms allows clinicians and readers alike to move beyond surface-level biomarkers and toward earlier, more precise metabolic risk detection.
This review synthesizes recent mechanistic, translational, and clinical literature published between 2024 and 2025, prioritizing studies that explore causality, pathway-level biology, and therapeutic relevance. Wherever possible, complex biochemical concepts have been translated into clinically meaningful insights without sacrificing scientific rigor.
Finally, this work reflects a broader philosophy in metabolic medicine: nutrition cannot be separated from metabolism, and metabolism cannot be understood without context. As precision medicine evolves, individualized amino acid metabolism—particularly BCAA handling—may become as central to preventive care as glucose, lipids, or blood pressure. I hope that this article encourages critical thinking, informed clinical application, and a more nuanced understanding of metabolic health.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Time-Restricted Eating: Metabolic Advantage or Just Fewer Calories? | DR T S DIDWAL
Exercise and Longevity: The Science of Protecting Brain and Heart Health as You Age | DR T S DIDWAL
Light and Longevity: Can Sunlight Slow Cellular Aging? | DR T S DIDWAL
References
Choi, B. H., Hyun, S., & Koo, S. H. (2024). The role of BCAA metabolism in metabolic health and disease. Experimental & Molecular Medicine, 56, 1552–1559. https://doi.org/10.1038/s12276-024-01263-6
Costa, M. V. L., Da Silva, L., Magalhães, M. P., Gomes De Jesus, T. H., De Araújo Oliveira, A., Arouche De Asevedo, L., Pereira, A. M. V., Cutrim, S. L. M., De Souza, J. G. S., & Caldas, A. S. (2025). BCAAs in metabolism and clinical practice: An integrative literature review. IOSR Journal of Business and Management, 27(8, Ser. 7), 07–15. https://doi.org/10.9790/487X-2708070715
Mansoori, S., Ho, M. Y., Ng, K. K., & Cheng, K. K. (2025). Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 26(2), e13856. https://doi.org/10.1111/obr.13856
Yin, Y., Li, H., Hu, Q., et al. (2025). BCAA metabolic dyshomeostasis in cardiovascular disease: Pathogenic mechanisms and intervention. American Journal of Cardiovascular Drugs. https://doi.org/10.1007/s40256-025-00775-4
Zhou, Y., Kou, J., Li, W., Wang, Y., Su, X., & Zhang, H. (2025). BCAA metabolism in cancer progression and therapy resistance: The balance between fuel and cell signaling. Frontiers in Pharmacology, 16. https://doi.org/10.3389/fphar.2025.1595176