Uric Acid and Cardiovascular Aging: An Emerging Link in Metabolic Health
Uric acid isn’t just about gout. New 2025 research links it to inflammation, metabolic disease, and cancer—learn how to lower your risk
METABOLISM
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/18/202612 min read

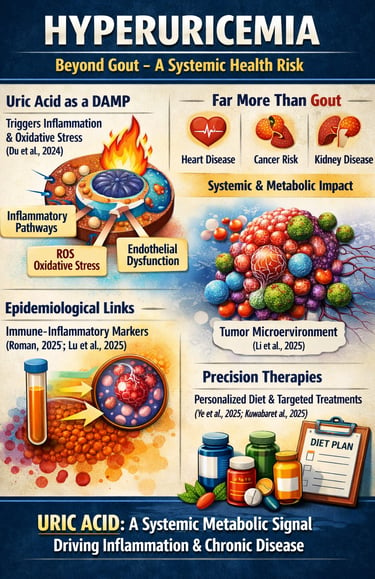
Hyperuricemia is no longer a biochemical footnote confined to gout clinics—it is emerging as a central player in systemic inflammation, metabolic dysfunction, and chronic disease risk. Traditionally defined as serum uric acid levels above 6.8 mg/dL, hyperuricemia was viewed primarily as a crystal deposition disorder. However, contemporary research reveals that soluble uric acid itself functions as a biologically active signaling molecule. Experimental and translational data show that uric acid acts as a danger-associated molecular pattern (DAMP), activating innate immune pathways, oxidative stress signaling, and endothelial dysfunction even in the absence of crystal formation (Du et al., 2024). Large-scale epidemiological analyses further demonstrate strong correlations between serum uric acid and systemic immune-inflammatory markers, reframing hyperuricemia as a driver—not merely a bystander—of chronic inflammation (Roman, 2025; Lu et al., 2025).
Beyond rheumatology, elevated uric acid is increasingly associated with cardiometabolic disease, hypertension, chronic kidney disease, and emerging oncologic pathways. Mechanistic evidence suggests that uric acid metabolism may influence tumor microenvironment remodeling through inflammatory transformation processes (Li et al., 2025). Meanwhile, precision-focused therapeutic strategies are evolving, emphasizing phenotype-based dietary and metabolic interventions alongside pharmacologic urate lowering (Ye et al., 2025; Kuwabara et al., 2025).
In 2025, understanding uric acid metabolism is essential for clinicians, researchers, and health-conscious individuals alike. Hyperuricemia represents a systemic metabolic signal—one that bridges inflammation, immunity, and chronic disease risk—demanding a broader, evidence-based approach to prevention and management.
Clinical Pearls
1. The "Silent Driver" of Inflammation
Scientific Perspective: Uric acid functions as a Danger-Associated Molecular Pattern (DAMP). Even when it isn't forming crystals in a joint, soluble urate can activate the NLRP3 inflammasome, triggering a systemic cascade of cytokines like IL-beta
Think of high uric acid like high blood pressure or "silent" cholesterol. You might not feel a "gout attack," but the high levels are still irritating your blood vessels and immune system behind the scenes, potentially leading to long-term fatigue or cardiovascular "wear and tear."
2. The Pan-Immune-Inflammation Value (PIV)
Scientific Perspective: Modern risk stratification should move beyond a simple serum uric acid (SUA) test. The PIV (calculated using neutrophils, platelets, monocytes, and lymphocytes) provides a bidirectional view of how hyperuricemia is affecting the total immune environment (Lu et al., 2025).
Your "uric acid number" is only half the story. To see the full picture of your health, your doctor might look at a standard Complete Blood Count (CBC) to see if your immune system is "on high alert." If both your uric acid and your immune markers are high, it’s a stronger signal to take action.
3. Metabolic Phenotyping vs. "The Gout Diet"
Scientific Perspective: The 2025 Ye et al. meta-analysis confirms that a "low-purine diet" is often insufficient. Hyperuricemia is frequently a symptom of insulin resistance. Elevated insulin reduces the kidneys' ability to excrete uric acid, making metabolic health more important than just avoiding steak or shellfish.
It’s not just about what you stop eating (like red meat), but how your body processes energy. Improving your blood sugar and losing visceral fat can often lower uric acid more effectively than a restrictive, miserable diet ever could.
4. The 6.8 mg/dL "Saturation Ceiling"
Scientific Perspective: 6.8 mg/dL is the physicochemical limit for urate solubility at normal body temperature. However, "sub-clinical" hyperuricemia—levels between 6.0 and 6.8 mg/dL—is increasingly linked to hypertension and renal microvascular damage (Kuwabara et al., 2025).
"Normal" on a lab report isn't always "Optimal." If your level is 6.to7 mg/dl, you might not have gout, but you are at the "speed limit" for what your blood can hold. Aiming for a "buffer zone" typically below 6.0 mg/dLoffers better protection for your heart and kidneys.
5. The Oncological Connection
Scientific Perspective: Emerging research (Li et al., 2025) suggests that uric acid metabolism can alter the tumor microenvironment. High levels may promote an "inflammatory transformation" that makes it easier for certain cancers to progress or evade the immune system.
Managing uric acid is no longer just about avoiding a painful toe—it’s a component of longevity and cancer prevention. Keeping your levels in check helps maintain a "clean" internal environment where healthy cells thrive and abnormal cells struggle to grow.
6. Precision Supplementation (The "Synergy" Effect)
Scientific Perspective: Network meta-analyses suggest that single-agent interventions (like just taking Vitamin C) are less effective than "multi-pathway" approaches. Combining xanthine oxidase inhibition (natural or pharmaceutical) with agents that improve oxidative stress markers provides superior outcomes.
Don't look for a "magic pill." The best results come from a "stack"—for example, combining a specific supplement (like tart cherry or Vitamin C) with better hydration and regular movement. This hits the uric acid from three different angles: production, excretion, and inflammation.
What is Hyperuricemia and Why Should You Care?
Hyperuricemia occurs when uric acid concentrations in your blood exceed normal levels (typically above 6.8 mg/dL). While some people with elevated uric acid remain asymptomatic, others develop debilitating conditions like gout or experience silent inflammation affecting multiple organ systems.
The emerging research landscape shows that serum uric acid is far more than a gout trigger—it's a potential biomarker for systemic inflammation and metabolic dysfunction (Du et al., 2024). Understanding uric acid mechanisms and the relationship between uric acid and inflammation has become crucial for preventive health strategies.
Asymptomatic Hyperuricemia
Asymptomatic hyperuricemia refers to persistently elevated serum uric acid levels without overt gout or kidney stones. Despite the absence of symptoms, growing evidence suggests it is not biologically benign. Elevated uric acid can drive subclinical inflammation, endothelial dysfunction, and metabolic risk even before crystal deposition occurs. Current consensus does not support universal pharmacologic treatment, but risk stratification, lifestyle intervention, and regular monitoring are recommended—especially in individuals with cardiometabolic disease, hypertension, or chronic kidney disease.
Recent Research Breakthrough: Du et al. (2024) on Hyperuricemia Mechanisms
The landmark review by Du and colleagues published in Signal Transduction and Targeted Therapy provides the most comprehensive overview of hyperuricemia mechanisms to date. This seminal work examines how uric acid-related diseases develop and progresses through the latest therapeutic advances in managing elevated uric acid (Du et al., 2024).
Key Findings:
Uric acid metabolism involves complex enzymatic pathways that, when disrupted, trigger systemic effects
Hyperuricemia mechanisms extend beyond crystalline deposition, affecting cellular signaling and immune responses
Therapeutic approaches range from traditional xanthine oxidase inhibitors to emerging targeted therapies
The relationship between uric acid and various chronic diseases requires personalized intervention strategies
This research establishes that managing hyperuricemia effectively demands understanding both the biochemical foundations and the broader metabolic context in which elevated serum uric acid operates.
The Systemic Connection: Roman (2025) Reveals Immune Inflammation and Gout Links
Roman's 2025 investigation, based on comprehensive NHANES data, demonstrates the critical relationship between systemic immune inflammation, serum uric acid levels, and gout development. This epidemiological analysis connects individual biomarkers to population-level disease patterns (Roman, 2025).
Critical Insights:
Serum uric acid strongly correlates with markers of systemic inflammation
The immune inflammation pathway appears central to gout manifestation
Uric acid elevation may drive inflammatory cascades independent of crystallization
Population-level data supports interventions targeting inflammatory responses alongside urate-lowering strategies
This research reframes gout from a simple crystalline arthropathy to a condition rooted in systemic immune dysfunction, suggesting that anti-inflammatory approaches should accompany traditional urate management.
Pan-Immune Inflammation and Uric Acid: Lu et al. (2025) Study
Lu and colleagues present groundbreaking evidence in Frontiers in Endocrinology examining the pan-immune-inflammation value (PIV) and its association with serum uric acid. This cross-sectional and longitudinal analysis reveals dynamic relationships between inflammatory indices and uric acid metabolism (Lu et al., 2025).
Major Takeaways:
Pan-immune-inflammation markers show bidirectional associations with serum uric acid levels
Longitudinal data indicates that elevated uric acid may predict future inflammatory progression
The inflammatory index captures immune dysfunction patterns linked to hyperuricemia more comprehensively than single markers
Understanding these inflammatory associations enables better risk stratification for uric acid-related complications
This study emphasizes that systemic inflammation and serum uric acid operate within interconnected networks, suggesting that interventions must address both parameters simultaneously.
Uric Acid as a Cancer Risk Factor: Li et al. (2025) Meta-Analysis
Perhaps most remarkably, Li and colleagues in the Journal of Advanced Research reveal that serum uric acid plays a pivotal role in inflammatory transformation of cancer. This research connects uric acid metabolism to oncological progression through inflammatory pathways (Li et al., 2025).
Essential Findings:
Elevated serum uric acid correlates with inflammatory markers implicated in cancer initiation and progression
Uric acid metabolism influences tumor microenvironments through immune cell activation
Hyperuricemia may represent an overlooked cancer risk factor requiring clinical attention
Managing serum uric acid levels could potentially reduce cancer-related inflammation
This paradigm-shifting work suggests that uric acid control may have implications far beyond gout management, potentially contributing to cancer prevention strategies.
Dietary Interventions and Uric Acid: Ye et al. (2025) Network Meta-Analysis
Ye and colleagues conducted an extensive network meta-analysis examining 13 dietary interventions for modulating uric acid levels, oxidative stress, and lipid metabolism in Nutrition & Metabolism. This comprehensive analysis synthesizes evidence on dietary supplements effectiveness (Ye et al., 2025).
Key Evidence-Based Findings:
Specific dietary supplements demonstrate varying efficacy in uric acid reduction
Interventions simultaneously address oxidative stress and lipid metabolism dysfunction
Certain supplement combinations show synergistic effects on serum uric acid lowering
Individual responses to dietary interventions vary significantly based on metabolic phenotypes
The evidence quality supports personalized dietary approaches rather than one-size-fits-all recommendations
This research validates that dietary management of hyperuricemia can be highly effective when properly targeted, offering patients evidence-based alternatives to pharmaceutical approaches.
Future Directions: Kuwabara et al. (2025) Hypertension Research Perspective
Kuwabara and colleagues provide critical perspective in Hypertension Research on current updates and future perspectives in uric acid research (Kuwabara et al., 2025). This forward-looking analysis identifies emerging research directions and unresolved questions.
Forward-Looking Insights:
Serum uric acid represents an underutilized biomarker in cardiovascular disease risk assessment
Emerging therapies targeting uric acid metabolism show promise beyond traditional agents
The relationship between uric acid and hypertension requires deeper investigation
Precision medicine approaches to hyperuricemia management are becoming increasingly feasible
Understanding tissue-specific uric acid effects remains a critical research frontier
This perspective emphasizes that uric acid research is rapidly evolving, with significant implications for clinical practice across multiple specialties.
Frequently Asked Questions About Hyperuricemia and Uric Acid
What Exactly is Uric Acid, and Where Does It Come From?
Uric acid is the final breakdown product of purine metabolism in humans. Purines come from dietary sources (red meat, organ meats, certain seafood) and are naturally produced within your body. When xanthine oxidase enzyme processes purines, uric acid is generated and normally excreted through your kidneys.
How Does Elevated Serum Uric Acid Lead to Disease?
Hyperuricemia damages health through multiple mechanisms. Uric acid crystals can deposit in joints, causing gout, but emerging research shows that uric acid molecules themselves trigger inflammatory responses in tissues throughout your body. Additionally, elevated serum uric acid impairs endothelial function, promotes oxidative stress, and appears to enhance cancer-promoting inflammation.
What's the Connection Between Uric Acid and Inflammation?
Recent studies reveal that uric acid acts as a danger-associated molecular pattern (DAMP), activating immune cells and inflammatory pathways. The pan-immune-inflammation value and other systemic inflammatory markers correlate closely with uric acid levels, suggesting bidirectional relationships where hyperuricemia drives inflammation and chronic inflammatory states elevate uric acid.
Can Diet Alone Lower Uric Acid Levels?
Absolutely. The network meta-analysis by Ye and colleagues demonstrates that dietary supplements and lifestyle modifications can significantly reduce serum uric acid. A uric acid-lowering diet emphasizing whole foods, limiting purines, and increasing hydration works effectively for many people, though individual responses vary considerably.
Is Gout the Only Consequence of Hyperuricemia?
No. While gout remains the most recognized hyperuricemia-related condition, emerging research links elevated serum uric acid to cardiovascular disease, metabolic syndrome, chronic kidney disease, and potentially cancer risk. This broader understanding necessitates managing hyperuricemia even in asymptomatic individuals.
What Are the Latest Treatment Options Beyond Allopurinol?
Modern hyperuricemia management includes xanthine oxidase inhibitors like allopurinol and febuxostat, uricosuric agents that increase urinary urate excretion, and uricase therapies. Emerging approaches target specific inflammatory pathways and address underlying metabolic dysfunction.
How Can I Know If I Have Hyperuricemia?
Hyperuricemia is diagnosed through a simple blood test measuring serum uric acid concentration. Normal levels are typically below 6.8 mg/dL, though this varies by laboratory. Asymptomatic individuals discovered to have elevated uric acid should consult healthcare providers about individual risk factors determining treatment necessity.
Should Everyone With High Uric Acid Levels Take Medication?
Not necessarily. Treatment decisions depend on uric acid levels, presence of gout symptoms, other risk factors, and individual preferences. The Du et al. (2024) review emphasizes that personalized approaches considering metabolic phenotypes are superior to uniform treatment protocols.
Hyperuricemia in 2025: From Crystal Disease to Systemic Signal
1️⃣ Hyperuricemia Is No Longer Just About Gout
For decades, serum uric acid was clinically relevant primarily when it crystallized. The 6.8 mg/dL solubility threshold defined pathology, and management focused on preventing monosodium urate deposition. That paradigm is now insufficient. Contemporary mechanistic data demonstrate that soluble uric acid exerts biologically active effects independent of crystallization, reshaping our understanding of hyperuricemia as a systemic metabolic signal rather than a purely rheumatologic disorder (Du et al., 2024).
2️⃣ Uric Acid as an Inflammatory Amplifier
Uric acid functions as a danger-associated molecular pattern (DAMP), activating innate immune pathways, oxidative stress cascades, and endothelial dysfunction. Activation of inflammasome pathways and downstream cytokine signaling reframes hyperuricemia as a contributor to chronic low-grade inflammation. This mechanistic shift is critical: inflammation may precede crystals, not the reverse.
3️⃣ The Cardiometabolic Bridge
Population-level analyses demonstrate strong associations between serum uric acid and systemic inflammatory indices, hypertension, insulin resistance, and vascular dysfunction (Roman, 2025; Lu et al., 2025). Whether uric acid is causal or amplificatory remains under investigation—but the signal is consistent. Elevated urate correlates with endothelial impairment, nitric oxide reduction, and oxidative stress, placing it squarely within the cardiometabolic risk continuum.
4️⃣ The Oncologic Frontier
Emerging evidence suggests that uric acid metabolism may influence tumor microenvironment remodeling via inflammatory transformation processes (Li et al., 2025). While causality is not established, the biological plausibility is compelling. Chronic inflammation, immune dysregulation, and oxidative stress represent common denominators linking hyperuricemia and malignancy. This expands the clinical conversation beyond joints and kidneys.
5️⃣ Asymptomatic Does Not Mean Benign
Asymptomatic hyperuricemia has long been considered clinically inert. That assumption deserves re-examination. Subclinical endothelial dysfunction and immune activation may occur well before gout manifests. Although universal pharmacologic treatment is not recommended, risk stratification—particularly in patients with cardiometabolic disease—has become essential.
6️⃣ Precision Over Purine Restriction
The era of blanket purine restriction is fading. Network meta-analytic evidence supports phenotype-based dietary and metabolic interventions that simultaneously target uric acid, oxidative stress, and lipid dysfunction (Ye et al., 2025). Hyperuricemia management is evolving toward precision medicine rather than dietary minimalism.
7️⃣ Therapeutic Evolution
Xanthine oxidase inhibitors remain foundational. However, emerging therapies and refined uricosuric strategies signal a broader therapeutic horizon. Future care may integrate inflammatory biomarker monitoring and individualized urate thresholds rather than rigid population cutoffs (Kuwabara et al., 2025).
8️⃣ A Systems Biology Perspective
Hyperuricemia intersects metabolism, immunity, vascular biology, and possibly oncogenesis. It exemplifies how a seemingly isolated biomarker participates in networked physiology. Treating it in isolation may miss its systemic implications.
9️⃣ The Cautionary Principle
Despite compelling data, hyperuricemia should not yet be labeled a universal causal agent of chronic disease. Many associations remain observational. Responsible clinical practice requires balanced interpretation—neither dismissive nor alarmist.
🔟 The Clinical Imperative
In 2025, serum uric acid should be interpreted contextually—alongside inflammatory indices, metabolic markers, renal function, and cardiovascular risk. Hyperuricemia represents a metabolic warning signal. The question is no longer whether it matters—but how broadly it matters, and for whom.
Uric acid is no longer merely a byproduct of purine metabolism. It is a biomarker at the intersection of inflammation and chronic disease. The future lies in precision, not reductionism.
Practical Recommendations for Managing Serum Uric Acid
Based on the collective evidence from these recent studies, consider these evidence-based strategies:
Lifestyle modifications should be your foundation: increase water intake, limit alcohol (especially beer), reduce purine-rich foods, maintain healthy weight, and engage in regular physical activity. These approaches address multiple mechanisms of hyperuricemia simultaneously.
Dietary supplements identified as effective in the Ye et al. meta-analysis may offer valuable adjuncts to lifestyle changes. Consider discussing evidence-based supplements with your healthcare provider.
Regular monitoring of serum uric acid levels enables early detection of hyperuricemia and assessment of intervention effectiveness.
Systemic inflammation assessment through comprehensive metabolic panels and inflammatory biomarkers provides context for understanding your individual risk profile.
Professional consultation with healthcare providers knowledgeable about modern uric acid management ensures personalized approaches reflecting your unique metabolic phenotype and health goals.
Author’s Note
Hyperuricemia has traditionally been viewed through a narrow clinical lens—primarily as the biochemical precursor to gout. However, the rapidly expanding body of research over the past decade, particularly between 2024 and 2025, challenges that reductionist view. Emerging mechanistic, epidemiological, and translational studies suggest that uric acid functions not merely as a metabolic waste product, but as a biologically active signaling molecule capable of influencing inflammation, endothelial function, oxidative stress, and systemic metabolic regulation.
As a clinician trained in internal medicine and deeply engaged in metabolic and cardiovascular research, my objective in writing this article was to synthesize evolving evidence into a clinically meaningful framework. The goal is not to overstate causality, but to highlight the growing recognition that hyperuricemia may act as both a biomarker and potential mediator of broader cardiometabolic and inflammatory risk.
Importantly, many associations described remain under active investigation. While mechanistic plausibility and population-level data are compelling, clinical decision-making must continue to rely on individualized risk assessment, established guidelines, and shared physician–patient discussion.
Science progresses through refinement, replication, and cautious interpretation. Uric acid research is currently in such a transitional phase—moving from organ-specific thinking toward systemic integration. I encourage readers to view hyperuricemia not with alarm, but with informed curiosity and evidence-based vigilance.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
Menopause and Heart Disease: Why Midlife Is a Cardiometabolic Turning Point | DR T S DIDWAL
How Insulin Resistance Accelerates Cardiovascular Aging | DR T S DIDWAL
Physical Activity, Adiposity, and Metabolic Health: What Science Reveals | DR T S DIDWAL
Sleep & Hypertension: Duration, Quality, and Blood Pressure | DR T S DIDWAL
Lower Blood Pressure Naturally: Evidence-Based Exercise Guide for Metabolic Syndrome | DR T S DIDWAL
References
Du, L., Zong, Y., Li, H., et al. (2024). Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduction and Targeted Therapy, 9, 212. https://doi.org/10.1038/s41392-024-01916-y
Kuwabara, M., Ae, R., Kosami, K., Kanbay, M., Andres-Hernando, A., Hisatome, I., & Lanaspa, M. A. (2025). Current updates and future perspectives in uric acid research, 2024. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 48(2), 867–873. https://doi.org/10.1038/s41440-024-02031-9
Li, Z., Su, Y., Su, H., Pan, J., Li, S., Lu, L., Ji, G., & Xu, H. (2025). Serum uric acid and its metabolism—A vital factor in the inflammatory transformation of cancer. Journal of Advanced Research, S2090-1232(25)00646-0. Advance online publication. https://doi.org/10.1016/j.jare.2025.08.031
Lu, Z., Chu, R., Wang, L., Cheng, Y., Gong, J., Huo, Y., & Sheng, C. (2025). Cross-sectional and longitudinal associations between pan-immune-inflammation value and serum uric acid. Frontiers in Endocrinology, 16. https://doi.org/10.3389/fendo.2025.1720450
Roman, Y. (2025). Correlation of systemic immune inflammation and serum uric acid with gout: Based on NHANES. Clinical Rheumatology, 44, 1393–1394. https://doi.org/10.1007/s10067-025-07329-8
Ye, G., Liu, C., Zheng, X., et al. (2025). The effectiveness and safety of specific dietary supplements in modulating uric acid levels, oxidative stress, and lipid metabolism in patients: A network meta-analysis of 13 interventions. Nutrition & Metabolism, 22, 80. https://doi.org/10.1186/s12986-025-00977-2