Sarcopenia and Metabolic Disorders: Understanding the Hidden Connection Between Muscle Loss and Metabolism
Explore how sarcopenia links to metabolic dysfunction, and learn evidence-based strategies to prevent muscle loss, boost metabolism, and age healthier
SARCOPENIA
DR T S DIDWAL MD
11/19/202517 min read
What if the key to healthy aging isn’t just your heart or bones—but your muscles? Sarcopenia, the silent loss of muscle mass, is more than a cosmetic concern—it’s a metabolic alarm bell signaling deep changes in your body’s energy systems. Discover how recent breakthroughs reveal that muscle health is central to metabolism, longevity, and quality of life
Clinical Pearls: Reclaiming Your Muscle Health
1. Muscle Loss is a Metabolic Emergency
Scientific Insight: Sarcopenia is more than just feeling weak; it's a sign of profound metabolic dysfunction, including mitochondrial decline (cellular energy failure) and chronic inflammation (inflammaging).
Patient-Friendly Translation: Your muscle is your metabolic engine. Losing it is like letting your car engine run inefficiently. We must fix the cellular problems first to truly restore strength.
2. The Power of Progressive Resistance Training (PRT)
Scientific Insight: PRT not only builds muscle mass but also actively reverses underlying metabolic damage, improving mitochondrial function and restoring metabolic flexibility (the ability to switch fuel sources efficiently).
Patient-Friendly Translation: Lifting weights is medicine. It's the most powerful signal you can give your body to repair its cells, improve your energy, and make your entire system metabolically younger. Aim for 2–3 challenging sessions per week.
3. Protein Quality is Non-Negotiable
Scientific Insight: Older muscles exhibit anabolic resistance, meaning they need more high-quality protein, especially the amino acid leucine (2.5–3g per meal), to trigger muscle protein synthesis effectively.
Patient-Friendly Translation: Spread your protein out, and make it count. Don't eat all your protein at dinner. You need a generous, high-quality dose (25–30g) at every main meal to "turn on" your muscle-building switch throughout the day.
4. It's Not Just What You Eat, It's What Your Cells Process
Scientific Insight: Recent research (Zuo et al., 2025) shows that sarcopenia involves a profound breakdown in the muscle's ability to catabolize (process) key nutrients like BCAAs and lipids. This leads to cellular toxicity and insulin resistance.
Patient-Friendly Translation: You might not be absorbing your nutrients correctly. We are fighting a problem inside your muscle cell. Training and specific nutrition (like Omega-3s and Vitamin D) help fix this cellular machinery so the food you eat can actually be used to build and repair.
5. Multi-Targeted Treatment Works Best
Scientific Insight: Because sarcopenia is multifaceted—involving metabolism, inflammation, and hormonal signaling—a comprehensive approach combining exercise, protein optimization, inflammation control, and targeted supplementation (Creatine, HMB, Vitamin D) yields superior results.
Patient-Friendly Translation: No single pill or exercise cures sarcopenia. We achieve the best results by creating a unified plan that tackles all aspects of the problem at once: Move, Nourish, Repair, and Recover.
What Is Sarcopenia? Beyond Simple Muscle Loss
Sarcopenia derives from the Greek words "sarx" (flesh) and "penia" (loss), but this clinical definition barely scratches the surface of its complexity. According to recent research, sarcopenia represents a multifactorial syndrome characterized by progressive skeletal muscle deterioration that accelerates with age (Boccardi, 2024).
The Clinical Reality
The numbers are staggering. Sarcopenia prevalence ranges from 10% to 27% in adults over 60 years old, with rates climbing dramatically as we age. But here's what makes sarcopenia particularly insidious: it doesn't just affect muscle tissue. As Ali et al. (2024) emphasize in their comprehensive review, sarcopenia triggers a cascade of metabolic alterations that impact multiple body systems, from glucose metabolism to lipid processing and protein synthesis.
The condition manifests through three primary characteristics:
Progressive loss of muscle mass—typically 3-8% per decade after age 30
Declining muscle strength—affecting both voluntary and involuntary muscle function
Reduced physical performance—impacting mobility, balance, and daily activities
What researchers are discovering, however, is that these physical manifestations are merely symptoms of deeper metabolic disturbances occurring at the cellular and molecular levels.
The Lipid Connection: How Fat Metabolism Drives Muscle Loss
One of the most fascinating revelations in recent sarcopenia research centers on lipid metabolism—how our bodies process and utilize fats. The groundbreaking study by Lal et al. (2025) published in BMC Biology provides compelling evidence that disrupted lipid metabolism isn't just associated with sarcopenia; it may be a primary driver of the condition.
Key Findings from the Lal Study
Lal and colleagues (2025) investigated the intricate relationship between lipid metabolism and age-related musculoskeletal disorders, including both sarcopenia and osteoporosis. Their research revealed several critical insights:
Lipid accumulation in muscle tissue: As we age, intramuscular lipid deposits increase dramatically. These aren't just passive accumulations—they actively interfere with muscle fiber function and contribute to insulin resistance within muscle cells. This phenomenon, sometimes called "muscle marbling" (similar to the marbling seen in aged beef), represents dysfunctional lipid storage that replaces healthy muscle tissue.
Altered fatty acid metabolism: The study demonstrated that aging muscle cells show impaired ability to oxidize (burn) fatty acids for energy. This metabolic inflexibility means muscles become less efficient at utilizing fat as fuel, leading to both energy deficits and toxic lipid accumulation.
Connection to osteoporosis: Perhaps most intriguingly, Lal et al. (2025) established clear links between lipid metabolic dysfunction and bone health. The same metabolic pathways that control muscle lipid processing also influence bone density, suggesting that sarcopenia and osteoporosis may share common metabolic origins.
Clinical Implications
Understanding the lipid-sarcopenia connection opens therapeutic possibilities. If abnormal lipid metabolism drives muscle loss, then interventions targeting fatty acid oxidation, lipid transport, or cellular energy metabolism could potentially slow or reverse sarcopenia progression.
Sarcopenia as a Metabolic Disease: The Multimodal Perspective
Boccardi's (2024) influential paper in Mechanisms of Ageing and Development challenges us to reconceptualize sarcopenia entirely. Rather than viewing it as simply a loss of muscle tissue, Boccardi proposes understanding sarcopenia as a complex metabolic disease requiring multimodal intervention.
The Metabolic Web
Boccardi (2024) identifies several interconnected metabolic disruptions characteristic of sarcopenia:
Mitochondrial dysfunction: The powerhouses of our cells—mitochondria—become less efficient with age. In sarcopenic muscle, mitochondria show reduced capacity for ATP production (cellular energy), increased production of damaging reactive oxygen species (ROS), and impaired quality control mechanisms. This mitochondrial decline directly compromises muscle cell survival and function.
Hormonal dysregulation: Sarcopenia involves complex changes in anabolic hormones (those that build tissue) and catabolic hormones (those that break down tissue). Declining levels of testosterone, growth hormone, and insulin-like growth factor 1 (IGF-1) combine with increased inflammatory signaling to shift the balance toward muscle breakdown.
Inflammatory environment: Chronic low-grade inflammation, termed "inflammaging," creates a hostile metabolic environment for muscle maintenance. Elevated levels of pro-inflammatory cytokines like IL-6 and TNF-α directly inhibit muscle protein synthesis while accelerating protein breakdown.
The Preventive and Regenerative Approach
What makes Boccardi's (2024) work particularly valuable is the emphasis on prevention and regeneration. The research advocates for a multimodal approach that includes:
Nutritional interventions targeting protein intake, amino acid supplementation, and antioxidant consumption
Exercise prescriptions combining resistance training and aerobic activity
Pharmacological strategies addressing hormonal deficiencies and inflammatory processes
Metabolic therapies aimed at restoring mitochondrial function and cellular energy production
This comprehensive framework recognizes that sarcopenia develops through multiple pathways and therefore requires multiple intervention strategies.
Advanced Detection and Metabolic Markers: The Ali Review
The comprehensive review by Ali et al. (2024) in the Canadian Journal of Physiology and Pharmacology provides a crucial update on sarcopenia detection methods and the metabolic alterations that characterize the condition.
Modern Diagnostic Approaches
Traditional sarcopenia diagnosis relied primarily on dual-energy X-ray absorptiometry (DEXA) scans and grip strength measurements. Ali and colleagues (2024) highlight emerging diagnostic tools that better capture the metabolic dimensions of sarcopenia:
Biomarker panels: Researchers are identifying specific blood biomarkers that reflect sarcopenic metabolism, including markers of protein turnover, inflammatory status, and oxidative stress. These biomarkers could enable earlier detection before significant muscle loss occurs.
Advanced imaging: Beyond measuring muscle quantity, new imaging techniques assess muscle quality by detecting intramuscular fat infiltration, muscle fiber composition, and tissue density—all indicators of metabolic health.
Functional metabolic testing: Assessments that measure how efficiently muscles utilize different fuel sources (glucose, fatty acids, amino acids) provide insights into the metabolic flexibility or rigidity of aging muscle tissue.
Metabolic Alterations in Sarcopenia
Ali et al. (2024) systematically catalog the metabolic changes accompanying sarcopenia progression:
Protein metabolism disturbances: Sarcopenic muscle shows both reduced protein synthesis rates and accelerated protein degradation. The balance tips dramatically toward catabolism, with the ubiquitin-proteasome system and autophagy-lysosome pathway becoming hyperactive.
Glucose metabolism impairment: Sarcopenic muscle exhibits pronounced insulin resistance, reducing glucose uptake and utilization. This creates a vicious cycle where muscles can't efficiently access glucose for energy, further compromising their function.
Energy metabolism deficits: Overall ATP production declines in sarcopenic muscle due to mitochondrial dysfunction, creating chronic energy deficits that limit muscle cell maintenance and regeneration.
Therapeutic Targets
The Ali review (2024) identifies several promising therapeutic targets emerging from metabolic research:
mTOR pathway modulators to enhance protein synthesis
AMPK activators to improve cellular energy sensing and metabolism
Mitochondrial-targeted antioxidants to reduce oxidative damage
Myostatin inhibitors to reduce signals that promote muscle breakdown
Aging-Related Sarcopenia: Metabolic Characteristics and Strategies
Chen and Wu's (2024) detailed analysis in Aging and Disease provides perhaps the most comprehensive synthesis of sarcopenia's metabolic characteristics and evidence-based therapeutic strategies.
The Metabolic Fingerprint of Sarcopenia
Chen and Wu (2024) describe sarcopenia's distinctive metabolic fingerprint—a pattern of biochemical changes that characterize the condition:
Decreased basal metabolic rate: Sarcopenic individuals show significantly reduced resting energy expenditure, primarily because muscle tissue is highly metabolically active. Less muscle means lower overall metabolic rate, contributing to weight gain and further metabolic dysfunction.
Altered substrate utilization: Healthy muscle tissue can flexibly switch between burning carbohydrates and fats depending on availability and activity level. Sarcopenic muscle loses this metabolic flexibility, becoming rigid in its fuel preferences and less efficient overall.
Dysregulated nutrient sensing: The molecular pathways that detect nutrient availability and coordinate appropriate metabolic responses—including the mTOR, AMPK, and insulin signaling pathways—become dysfunctional in sarcopenic muscle.
Evidence-Based Therapeutic Strategies
Chen and Wu (2024) provide a thorough evaluation of intervention strategies with proven metabolic benefits:
Nutritional interventions:
High-quality protein intake (1.2-1.5 g/kg body weight daily) with emphasis on leucine-rich proteins
Omega-3 fatty acids to modulate inflammation and support muscle metabolism
Vitamin D supplementation to address deficiency and support muscle function
Creatine supplementation to enhance muscle energy metabolism
Exercise prescriptions:
Progressive resistance training (2-3 sessions weekly) to stimulate muscle protein synthesis
High-intensity interval training to improve mitochondrial function and metabolic flexibility
Combined training protocols integrating strength and cardiovascular exercise
Pharmacological approaches:
Selective androgen receptor modulators (SARMs) showing promise in early trials
Ghrelin mimetics to stimulate appetite and anabolic signaling
Anti-inflammatory agents targeting chronic inflammation
The strength of Chen and Wu's (2024) work lies in its evidence-based approach, carefully evaluating which interventions show genuine metabolic benefits versus those lacking rigorous support.
The Role of Peptides: Molecular Messengers in Muscle Wasting
Naumovski et al.'s (2025) scoping review in the Journal of Cachexia, Sarcopenia and Muscle provides a fascinating exploration of how bioactive peptides influence skeletal muscle wasting—a perspective that bridges molecular biology and clinical intervention.
Peptides as Metabolic Regulators
Peptides—short chains of amino acids—function as crucial signaling molecules that regulate muscle metabolism. Naumovski and colleagues (2025) identify several peptide classes with significant roles in sarcopenia:
Anabolic peptides: Certain peptides directly stimulate muscle protein synthesis and promote muscle cell growth. Examples include fragments derived from dietary proteins that, when released during digestion, signal muscle cells to enhance their anabolic activity.
Anti-inflammatory peptides: Some bioactive peptides modulate inflammatory signaling pathways, potentially reducing the chronic inflammation that accelerates muscle loss. These peptides might work by interfering with pro-inflammatory cytokine production or enhancing anti-inflammatory responses.
Metabolic regulatory peptides: Various peptides influence how muscle cells sense and respond to nutrients, affecting glucose uptake, fatty acid oxidation, and overall energy metabolism.
Clinical and Nutritional Applications
The Naumovski review (2025) highlights the therapeutic potential of peptide-based interventions:
Dietary protein sources: Different protein sources release distinct peptide profiles during digestion. Foods rich in beneficial bioactive peptides—including dairy proteins (whey and casein), soy, and certain marine proteins—may offer advantages beyond their amino acid content.
Peptide supplementation: Specific isolated peptides or peptide mixtures could be developed as targeted supplements to address sarcopenia. This represents a more sophisticated approach than simple protein supplementation, targeting specific metabolic pathways.
Drug development: Understanding peptide signaling in muscle could inform the development of peptide-based pharmaceuticals that more precisely target sarcopenic mechanisms.
Key Takeaways from the Peptide Research
Naumovski et al. (2025) emphasize several important points:
Not all proteins are metabolically equivalent—their peptide profiles matter
The timing and context of protein/peptide consumption may influence their metabolic effects
Peptide-based interventions could complement traditional approaches to sarcopenia management
Further research is needed to translate peptide biology into practical clinical applications
The BCAA Breakthrough: Disrupted Amino Acid Metabolism as a Causal Mechanism
Perhaps the most exciting recent discovery comes from Zuo et al.'s (2025) landmark study in Nature Aging, which employs sophisticated multi-omic profiling to identify disrupted branched-chain amino acid (BCAA) catabolism as a causal mechanism in sarcopenia.
Multi-Omic Investigation
The Zuo study (2025) represents the cutting edge of sarcopenia research, integrating multiple layers of biological information:
Genomics: Analysis of genetic variations associated with sarcopenia risk Transcriptomics: Measurement of which genes are active in sarcopenic versus healthy muscle Proteomics: Comprehensive profiling of protein expression and modifications Metabolomics: Detailed mapping of small molecule metabolites, including amino acids, lipids, and energy substrates
This comprehensive approach revealed that BCAA metabolism—specifically the breakdown and utilization of the amino acids leucine, isoleucine, and valine—is profoundly disrupted in sarcopenia.
The BCAA Connection
Branched-chain amino acids (BCAAs) are essential nutrients critical for muscle metabolism. They serve multiple functions:
Direct building blocks for muscle protein synthesis
Signaling molecules that activate anabolic pathways (particularly through mTOR)
Substrate for energy production in muscle cells
Regulators of glucose metabolism and insulin sensitivity
Zuo et al. (2025) discovered that sarcopenic muscle shows impaired BCAA catabolism—the metabolic pathways that break down BCAAs. This impairment leads to:
BCAA accumulation: Paradoxically, sarcopenic muscle may have elevated BCAA levels not because of excessive intake, but because it cannot properly metabolize them. This accumulation can become toxic and interfere with other metabolic processes.
Metabolic inflexibility: Inability to properly catabolize BCAAs reduces muscle cells' ability to use these amino acids as alternative fuel sources, contributing to energy deficits.
Insulin resistance: Disrupted BCAA metabolism is linked to the development of muscle insulin resistance, further compromising glucose metabolism.
Therapeutic Implications
The identification of BCAA catabolism as a causal mechanism opens exciting therapeutic possibilities:
Targeted interventions: Rather than simply supplementing with more BCAAs, interventions could aim to restore proper BCAA metabolism by enhancing the enzymes and pathways responsible for BCAA catabolism.
Precision nutrition: Understanding individual BCAA metabolic capacity could enable personalized dietary recommendations. Some individuals might benefit from BCAA supplementation, while others might need interventions to enhance BCAA clearance.
Drug development: The specific enzymes involved in BCAA catabolism represent potential drug targets. Therapies that restore normal BCAA metabolism could address a root cause of sarcopenia rather than merely treating symptoms.
The Causal Link
What makes the Zuo study (2025) particularly powerful is its establishment of causality rather than mere association. Using sophisticated genetic analyses (Mendelian randomization), the researchers demonstrated that disrupted BCAA metabolism doesn't just correlate with sarcopenia—it appears to directly contribute to its development. This distinction is crucial for therapeutic development, as it identifies BCAA catabolism as a legitimate target for intervention rather than simply a consequence of muscle loss.
Integrating the Evidence: A Unified Metabolic Framework
When we synthesize findings from these six groundbreaking studies, a coherent metabolic framework for understanding sarcopenia emerges:
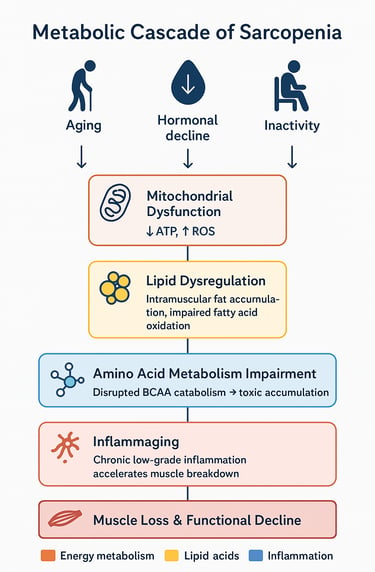
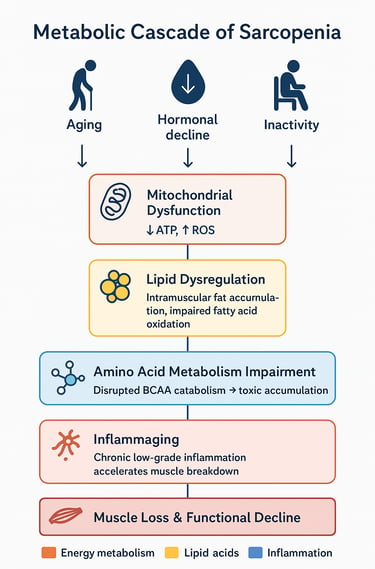
The Metabolic Cascade
Initial triggers: Aging, reduced physical activity, and hormonal changes initiate metabolic disruptions
Mitochondrial decline: Reduced cellular energy production compromises muscle maintenance
Lipid dysregulation: Abnormal fat accumulation and utilization further impair muscle cell function
Amino acid metabolism disruption: Particularly BCAA catabolism impairment creates toxic accumulations and energy deficits
Inflammatory environment: Chronic inflammation accelerates protein breakdown
Insulin resistance: Metabolic inflexibility worsens, creating glucose utilization problems
Muscle loss and dysfunction: The culmination of these metabolic disturbances manifests as sarcopenia
Common Metabolic Themes
Several metabolic themes consistently emerge across all the research reviewed:
Energy metabolism central role: Whether examining lipids, amino acids, or cellular powerhouses, energy metabolism dysfunction appears foundational to sarcopenia development.
Metabolic inflexibility: Healthy muscle tissue can adapt its fuel utilization to circumstances. Sarcopenic muscle loses this flexibility, becoming metabolically rigid and inefficient.
Multi-system involvement: Sarcopenia isn't isolated to muscle tissue—it involves disruptions in endocrine signaling, inflammatory responses, nutrient sensing, and cellular quality control across multiple tissues.
Reversibility potential: Importantly, many metabolic alterations in sarcopenia appear potentially reversible with appropriate interventions, offering hope for both prevention and treatment.
Key Scientific Concepts;
1. The BCAA Breakthrough (The New Muscle Secret)
Myth: Sarcopenia is just about needing more protein.
The New Metabolic Truth (Zuo et al. 2025): Sarcopenia isn't caused by a lack of BCAAs, but by a breakdown in BCAA metabolism inside the muscle cell. Aging muscle struggles to process these key amino acids, leading to toxic accumulation and dysfunction. This is a causal mechanism, not just a symptom.
Actionable Takeaway: Simply taking BCAA supplements might not be enough! Future treatments must focus on restoring the muscle's ability to metabolize amino acids, not just supplementing them. Resistance training remains the most powerful tool to improve this internal cellular machinery.
2. The Hidden Lipid Connection (Sarcopenia's Fat Problem)
Old View: Muscle loss is separate from fat gain.
The Metabolic Reality (Lal et al. 2025): Sarcopenia is driven by dysfunctional lipid metabolism. Fat doesn't just sit around the muscle; it infiltrates it ("muscle marbling"), actively interfering with cell function. This intramuscular fat accumulation contributes directly to insulin resistance and compromises the muscle's fuel efficiency.
Actionable Takeaway: To fight sarcopenia, you must fight metabolic inflexibility. Progressive resistance training and a diet targeting inflammation and overall fat metabolism are key to forcing muscles to burn fat more efficiently and restoring health.
3. Progressive Resistance Training (The Metabolic Restorer)
The Power of Exercise: Progressive Resistance Training (PRT) is more than a muscle builder—it's a metabolic therapy.
What PRT Does:
Restores Metabolic Flexibility: Helps the muscle efficiently switch between burning fats and carbohydrates.
Reactivates Mitochondria: Reverses age-related decline in the cell's energy powerhouses.
Improves BCAA Catabolism: Stimulates the enzymes necessary to properly process amino acids (Source: Zuo et al. 2025).
Conclusion: Exercise is not just about building muscle mass; it's about repairing the fundamental cellular machinery that goes wrong in sarcopenia.
Frequently Asked Questions (FAQs)
Q1: Can sarcopenia be reversed, or is it inevitable with aging?
While some age-related muscle decline is common, sarcopenia is not inevitable, and evidence suggests it can be partially reversed. The metabolic dysfunction underlying sarcopenia responds to interventions including resistance exercise, optimized nutrition, and in some cases, targeted pharmacological therapy. The key is early intervention—the earlier metabolic disturbances are addressed, the better the potential for reversal.
Q2: How much protein do I really need to prevent sarcopenia?
Research consistently suggests that older adults benefit from higher protein intakes than younger individuals—approximately 1.2-1.5 grams per kilogram of body weight daily, distributed across meals. For a 70 kg (154 lb) person, this translates to roughly 84-105 grams of protein daily. Importantly, protein quality matters; sources rich in leucine and bioactive peptides may offer additional metabolic benefits.
Q3: Are BCAA supplements beneficial for preventing sarcopenia?
The relationship is complex. While BCAAs, particularly leucine, stimulate muscle protein synthesis, recent research (including the Zuo et al., 2025 study) reveals that some individuals with sarcopenia actually have impaired BCAA metabolism. This suggests that simply adding more BCAAs might not be beneficial for everyone. A more nuanced approach considering individual metabolic capacity may be necessary. Consult with a healthcare provider before starting BCAA supplementation.
Q4: What type of exercise is most effective for sarcopenia?
Progressive resistance training targeting major muscle groups 2-3 times per week shows the strongest evidence for combating sarcopenia. However, combining resistance training with aerobic exercise provides additional metabolic benefits, including improved mitochondrial function and metabolic flexibility. The best exercise program is one that you can sustain long-term.
Q5: How early should I be concerned about sarcopenia?
Muscle mass typically begins declining around age 30, with accelerated loss after age 60. However, the metabolic changes predisposing to sarcopenia may begin even earlier. The best approach is proactive—maintaining muscle health through exercise and nutrition throughout adulthood rather than waiting for problems to emerge.
Q6: Can medications help with sarcopenia?
Currently, no medications are specifically approved for sarcopenia treatment, though several are in various stages of development. Some healthcare providers may recommend treating underlying conditions that contribute to muscle loss (such as vitamin D deficiency or hormonal imbalances). Future therapies targeting specific metabolic pathways identified in recent research show promise.
Q7: How is sarcopenia diagnosed?
Diagnosis typically involves assessing three components: muscle mass (via DEXA scan, BIA, or other imaging), muscle strength (typically grip strength), and physical performance (walking speed, chair stand tests). Some research centers are developing metabolic biomarker panels that may enable earlier detection of sarcopenic changes before significant muscle loss occurs.
Q8: Does sarcopenia affect men and women differently?
Yes, there are sex differences in sarcopenia development and progression. Men typically have greater muscle mass initially but may experience more rapid decline. Women face additional challenges related to menopause-associated hormonal changes that can accelerate muscle loss. However, the fundamental metabolic mechanisms appear similar across sexes, and intervention strategies are generally effective for both men and women.
Key Takeaways: Essential Points to Remember
🔹 Sarcopenia is fundamentally a metabolic disease, not simply a loss of muscle tissue. Understanding its metabolic origins opens new avenues for prevention and treatment.
🔹 Lipid metabolism dysfunction plays a central role in sarcopenia, with abnormal fat accumulation in muscle tissue and impaired fatty acid utilization contributing significantly to muscle deterioration.
🔹 Disrupted BCAA catabolism has been identified as a causal mechanism in sarcopenia development, representing a specific metabolic target for future interventions.
🔹 Mitochondrial dysfunction underlies much of the energy metabolism problems in sarcopenic muscle, affecting the cellular powerhouses that generate ATP.
🔹 Chronic low-grade inflammation (inflammaging) creates a hostile metabolic environment that accelerates muscle protein breakdown while inhibiting synthesis.
🔹 Multimodal interventions are most effective—combining nutritional optimization, progressive resistance exercise, and addressing underlying metabolic dysfunction provides the best outcomes.
🔹 Bioactive peptides from dietary proteins may offer benefits beyond their amino acid content, influencing muscle metabolism through signaling mechanisms.
🔹 Early intervention matters—the metabolic changes preceding visible muscle loss may be more easily reversed than established sarcopenia, emphasizing the importance of proactive muscle health maintenance.
🔹 Personalization is increasingly important—individual variations in metabolic dysfunction suggest that tailored approaches based on specific metabolic profiles may optimize outcomes.
🔹 Prevention is possible—sarcopenia is not an inevitable consequence of aging. Evidence-based strategies can maintain muscle health and metabolic function well into advanced age.
Conclusion: A New Era in Understanding Muscle Health
The research published in 2024 and 2025 marks a transformative period in sarcopenia science. We've moved beyond viewing muscle loss as a simple, inevitable consequence of aging to understanding it as a complex metabolic disorder with identifiable mechanisms and modifiable risk factors.
The convergence of evidence from lipid metabolism (Lal et al., 2025), comprehensive metabolic profiling (Boccardi, 2024; Ali et al., 2024; Chen & Wu, 2024), peptide signaling (Naumovski et al., 2025), and amino acid metabolism (Zuo et al., 2025) paints a remarkably coherent picture: sarcopenia emerges from interconnected metabolic disruptions that affect how muscle cells produce energy, process nutrients, and maintain themselves.
This metabolic framework is not merely of academic interest—it has profound practical implications. By targeting specific metabolic pathways, we can develop more effective interventions. By understanding individual metabolic variations, we can personalize treatment approaches. By detecting metabolic changes early, we can intervene before significant muscle loss occurs.
Perhaps most importantly, this research empowers us with actionable knowledge. We don't need to passively accept muscle decline as an inevitable part of aging. Through evidence-based nutritional strategies, appropriate exercise, and emerging therapeutic approaches, we can maintain muscle health and metabolic function far longer than previous generations imagined possible.
The journey from understanding sarcopenia's metabolic origins to implementing effective population-wide prevention and treatment strategies continues. But the foundation laid by this recent research provides a solid platform for progress. As we stand at this exciting juncture, the future of muscle health in aging populations looks increasingly bright.
Call to Action: Take Charge of Your Muscle Health Today
Understanding sarcopenia's metabolic basis is powerful—but knowledge only transforms lives when translated into action. Here's how you can apply these insights:
Assess your current status: Consider scheduling a comprehensive health evaluation that includes muscle mass assessment, strength testing, and metabolic screening.
Optimize your nutrition: Review your protein intake and distribution across meals. Are you consistently consuming 1.2-1.5 g/kg of high-quality protein daily? Consider incorporating omega-3-rich foods and ensuring vitamin D adequacy.
Start or enhance your exercise routine: If you're not currently doing resistance training, begin with a qualified fitness professional who can design an age-appropriate program. If you're already exercising, ensure your routine adequately challenges your muscles.
Address underlying health issues: Work with your healthcare provider to optimize management of conditions like diabetes, inflammation, or hormonal imbalances that could accelerate muscle loss.
Stay informed: The pace of sarcopenia research is accelerating. Follow reputable sources for updates on new interventions and evidence-based recommendations.
Join the Conversation
Sarcopenia affects millions but remains underrecognized. Share this information with family members, friends, and colleagues who might benefit. Discuss muscle health with your healthcare providers. Support research and policy initiatives focused on healthy aging.
Your muscles are more than just the machinery of movement—they're metabolic organs central to your overall health and quality of life. Taking action to understand and preserve your muscle health is one of the most important investments you can make in your future wellbeing.
The metabolic revolution in sarcopenia understanding has arrived. The question now is: how will you use this knowledge to transform your health or the health of those you serve?
Related Articles
Vitamin D Deficiency and Sarcopenia: The Critical Connection | DR T S DIDWAL
Sarcopenia in Diabetes: Managing Muscle Loss with Chronic Disease | DR T S DIDWAL
How to Prevent Sarcopenia: Fight Age-Related Muscle Loss and Stay Strong | DR T S DIDWAL
Who Gets Sarcopenia? Key Risk Factors & High-Risk Groups Explained | DR T S DIDWAL
Sarcopenia: The Complete Guide to Age-Related Muscle Loss and How to Fight It | DR T S DIDWAL
Best Exercises for Sarcopenia: Strength Training Guide for Older Adults | DR T S DIDWAL
Best Supplements for Sarcopenia: Vitamin D, Creatine, and HMB Explained | DR T S DIDWAL
References
Ali, S. R., Nkembo, A. T., Tipparaju, S. M., Ashraf, M., & Xuan, W. (2024). Sarcopenia: Recent advances for detection, progression, and metabolic alterations along with therapeutic targets. Canadian Journal of Physiology and Pharmacology, 102(12), 697–708. https://doi.org/10.1139/cjpp-2024-0201
Boccardi, V. (2024). Sarcopenia: A dive into metabolism to promote a multimodal, preventive, and regenerative approach. Mechanisms of Ageing and Development, 219, 111941. https://doi.org/10.1016/j.mad.2024.111941
Chen, Y., & Wu, J. (2024). Aging-related sarcopenia: Metabolic characteristics and therapeutic strategies. Aging and Disease, 16(2), 1003–1022. https://doi.org/10.14336/AD.2024.0407
Lal, S., Gunji, S., Ahluwalia, P., Patil, R., Singh, S., Akbare, S., Limaye, S., Vishwanatha, J. K., & Banerji, D. (2025). Lipid metabolism in age-related musculoskeletal disorders: Insights into sarcopenia and osteoporosis. BMC Biology, 23, 265. https://doi.org/10.1186/s12915-025-02383-9
Naumovski, P., De Spiegeleer, B., Wakjira, A., Van De Wiele, C., Mouly, V., Goljanek‐Whysall, K., Da Costa, K. S., De Oliveira, E. C. L., Wynendaele, E., & De Spiegeleer, A. (2025). Role of peptides in skeletal muscle wasting: A scoping review. Journal of Cachexia, Sarcopenia and Muscle, 16(6), e70109. https://doi.org/10.1002/jcsm.70109
Zuo, X., Zhao, R., Wu, M., Wang, M., Zhao, Z., Jia, S., Sun, L., Qi, Y., Fan, W., Ruan, Q., Zhang, X., Deng, L., Song, W., Zou, L., Yan, M., Tian, Q., Jin, Y., Yu, Z., & Shen, B. (2025). Multi-omic profiling of sarcopenia identifies disrupted branched-chain amino acid catabolism as a causal mechanism and therapeutic target. Nature Aging, 5, 419–436. https://doi.org/10.1038/s43587-024-00797-8
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with qualified healthcare professionals before making changes to your health regimen or starting new treatments.