Muscle Quality vs. Muscle Mass: Why Strength Per Pound Predicts Healthy Aging and Longevity
Discover why muscle quality and strength per pound predict healthy aging, longevity, and metabolic health better than muscle mass alone.
AGINGEXERCISE
Dr. T.S. Didwal, M.D.(Internal Medicine)
2/21/202616 min read
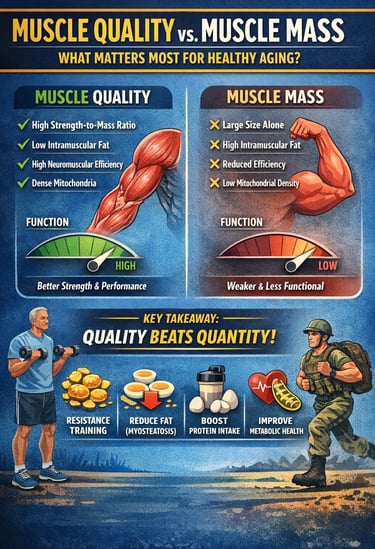
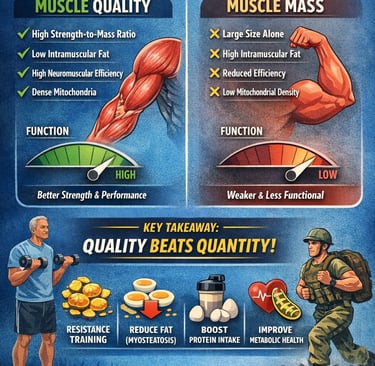
Muscle quality — not just muscle mass — is emerging as the most powerful predictor of healthy aging, physical performance, and long-term metabolic resilience. While traditional definitions of sarcopenia have emphasized declining skeletal muscle mass, recent research shows that the functional capacity of muscle tissue — its ability to generate force efficiently — matters far more than size alone.
Studies now demonstrate that individuals with higher strength-to-mass ratios consistently outperform those with larger but lower-quality muscles. For example, muscle quality independently predicts muscle strength and Short Physical Performance Battery (SPPB) outcomes even after adjusting for age, BMI, and type 2 diabetes mellitus (T2DM) (Kim et al., 2025). Similarly, narrative and systematic reviews highlight that intramuscular fat infiltration (myosteatosis), reduced neuromuscular efficiency, altered muscle architecture, and declining mitochondrial density all impair muscle function despite preserved mass (Kuschel et al., 2022; Rizka et al., 2025).
Importantly, muscle quality is not only relevant in older adults. It also serves as a key biomarker of athletic performance, military readiness, and functional capacity in young populations (Naimo et al., 2021). Across age groups, resistance training, aerobic conditioning, and optimized protein intake improve muscle morphology, neural activation, and metabolic efficiency — directly enhancing muscle quality.
The emerging consensus is clear: quantity without quality is insufficient. For clinicians, exercise professionals, and researchers, shifting focus toward measurable indices of muscle function, specific force, and echo intensity may redefine how we assess frailty, prevent disability, and optimize performance across the lifespan (Kim et al., 2025; Kuschel et al., 2022; Naimo et al., 2021; Rizka et al., 2025).
Clinical Pearls
The "Marbleized" Muscle (Myosteatosis)
Intramuscular adipose tissue (IMAT) is a primary driver of reduced specific force. Increased echo intensity on ultrasound reflects the replacement of contractile tissue with non-contractile lipid deposits, directly impairing lateral force transmission and metabolic signaling.
Think of your muscle like a steak. We want a "lean cut," not a "highly marbled" one. Even if your arms or legs look the same size, if fat starts "marbling" into the muscle fibers, the muscle becomes weaker and less efficient. We want to train to keep the muscle "clean" and strong.
2. Strength-to-Mass Ratio vs. Absolute Mass
Sarcopenia diagnosis should prioritize the Muscle Quality Index (MQI)—the ratio of torque or grip strength to appendicular skeletal muscle mass (ASM)—rather than lean mass alone. High ASM does not preclude functional frailty if the strength-to-mass ratio is sub-optimal.
It’s not about how much muscle you have; it’s about how much "horsepower" you get out of every pound of that muscle. A smaller, high-quality engine often outperforms a large, rusty one. Focus on how much you can lift or move, not just the number on a body composition scale.
3. The "Neural Spark" (Neuromuscular Drive)
Muscle quality is heavily dependent on motor unit recruitment, discharge frequency, and synchronization. Age-related power loss often stems from neural "denervation" of Fast-Twitch (Type II) fibers before physical atrophy is fully visible on a DXA scan.
Your muscles are like lightbulbs, but your nerves are the wiring. You can have a brand-new bulb (mass), but if the wiring is frayed (nerves), the light won't be bright. Exercises that require speed or coordination "re-wire" your system to keep the lights on bright.
4. The Metabolic Buffer in Diabetes
In Type 2 Diabetes (T2DM), muscle quality serves as a modifiable buffer against metabolic decline. High-quality muscle maintains better insulin sensitivity and mitochondrial density, which can attenuate the functional "performance gap" typically seen in diabetic cohorts.
For those with diabetes, muscle is your "sugar sponge." High-quality muscle is much better at soaking up blood sugar and turning it into energy. Improving the quality of your muscle can actually protect you from the typical fatigue and weakness associated with the disease.
5. Architectural Integrity (Pennation Angle)
Muscle quality includes morphological factors such as the pennation angle and fascicle length. These structural arrangements dictate the physiological cross-sectional area (PCSA) and the muscle's ability to generate maximal force per unit of volume.
The way your muscle fibres are "angled" within your body affects how strong you are. Think of it like the bristles on a broom; if they are angled correctly, they can move more dirt. Strength training ensures your "internal architecture" stays built for power.
6. The "Golden Opportunity" in Youth
Peak muscle quality is a longitudinal biomarker. Optimising Type II fibre cross-sectional area and mitochondrial oxidative capacity in the second and third decades of life creates a "reserve" that delays the onset of clinical frailty in later life.
Think of your muscle quality like a retirement savings account. The "deposits" of high-intensity training and good protein you make in your 20s and 30s pay interest for the rest of your life. It is much easier to maintain high-quality muscle than it is to rebuild it from scratch later.
Rethinking What "Strong" Really Means
What Is Muscle Quality? Defining a Complex Construct
Before diving into the research, it is worth establishing what "muscle quality" actually means, because the term is used in subtly different ways across disciplines.
At its most fundamental level, muscle quality refers to the functional output of a given amount of muscle tissue. In clinical and research contexts, it is most commonly operationalized as the ratio of muscle strength (typically grip strength or knee extensor torque) to muscle mass or cross-sectional area. A person with high muscle quality generates more force per unit of muscle tissue than someone with low muscle quality, even if both individuals have the same absolute mass.
However, as Kuschel et al. (2022) clarify in their systematic literature review, muscle quality is a multidimensional construct that encompasses not just this strength-to-mass ratio but also neuromuscular efficiency, muscle architecture (including pennation angle, fascicle length, and physiological cross-sectional area), fiber type composition, intramuscular fat infiltration, connective tissue content, metabolic capacity, and mitochondrial density. These structural and biochemical properties collectively determine how effectively a muscle converts neural input and metabolic energy into mechanical force and movement.
Understanding muscle quality as a multifactorial construct, rather than a single metric, is critical to appreciating why its measurement and optimization are both complex and clinically meaningful.
Study 1: Muscle Quality as the Cornerstone of Sarcopenia Diagnosis
Rizka, Pohan, and Andrianie (2025) published a compelling narrative review in Experimental Gerontology arguing that current sarcopenia diagnostic frameworks — which rely heavily on muscle mass measured via dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) — are fundamentally incomplete. The authors review evidence from multiple cohort studies and meta-analyses to demonstrate that muscle mass alone is a poor predictor of functional outcomes in older adults. Two individuals can have identical muscle mass but dramatically different physical performance outcomes depending on the quality of that muscle.
The review examines how intramuscular fat infiltration — sometimes called myosteatosis — increases with age, disease, and physical inactivity, effectively replacing contractile tissue with non-contractile lipid deposits without necessarily changing overall muscle volume. This phenomenon explains why some older adults who appear "normal" on mass-based assessments still suffer falls, weakness, and disability.
Rizka et al. (2025) advocate for the incorporation of muscle quality indices — including echo intensity on ultrasound (a surrogate for intramuscular fat), muscle echo intensity, and specific force measures — into standard sarcopenia diagnostic protocols alongside existing criteria from frameworks like the European Working Group on Sarcopenia in Older People (EWGSOP2) and the Asian Working Group for Sarcopenia (AWGS).
Key Takeaway
The central message of Rizka et al. (2025) is that sarcopenia diagnosis must evolve from a quantity-based to a quality-informed paradigm. Relying exclusively on muscle mass misclassifies a substantial proportion of older adults who are functionally impaired despite appearing muscularly adequate on conventional scans. Integrating muscle quality assessments into diagnostic criteria could improve early identification of at-risk individuals and enable more targeted, effective interventions.
Study 2: Muscle Quality Outperforms Mass in Predicting Strength and Performance
Kim et al. (2025) conducted a large observational study published in the Journal of Cachexia, Sarcopenia and Muscle examining the independent contributions of muscle quality, muscle mass, and diabetes status to muscle strength and physical performance in a community-based Korean cohort. Their study is particularly notable for its inclusion of individuals with type 2 diabetes mellitus (T2DM), a population at elevated risk for sarcopenia and functional decline due to chronic inflammation, insulin resistance, and neuropathy.
Using data from participants in the Korean Genome and Epidemiology Study, the authors assessed muscle mass via BIA, muscle strength via handgrip dynamometry, and physical performance via the Short Physical Performance Battery (SPPB). Muscle quality was operationalized as the ratio of handgrip strength to appendicular skeletal muscle mass (ASM). The investigators employed multiple regression models to disentangle the relative contributions of each variable while controlling for age, sex, BMI, and comorbidity burden.
Their findings were striking. Muscle quality was significantly and independently associated with both muscle strength and physical performance, even after controlling for absolute muscle mass and diabetes status. Crucially, participants with high muscle quality but relatively lower muscle mass outperformed those with high muscle mass but lower quality on functional assessments. Furthermore, the detrimental effects of diabetes on physical performance were substantially attenuated in individuals who maintained good muscle quality, suggesting that muscle quality may serve as a modifiable buffer against the functional consequences of metabolic disease.
Key Takeaway
Kim et al. (2025) provide robust epidemiological evidence that muscle quality is a stronger predictor of muscle strength and physical performance than muscle mass alone, even in the context of metabolic disease. For clinicians managing patients with diabetes or cardiometabolic risk, this study suggests that treatment goals should explicitly include the preservation and improvement of muscle quality — not just the prevention of muscle mass loss — as a strategy for maintaining functional independence.
Study 3: The Multifactorial Architecture of Muscle Quality
The systematic literature review by Kuschel, Sonnenburg, and Engel (2022), published in Healthcare, provides the most comprehensive conceptual map of muscle quality to date. Analyzing dozens of peer-reviewed studies, the authors identify and categorize the major structural, neural, metabolic, and biochemical determinants of muscle strength — the primary functional output used to index muscle quality — and evaluate the strength of evidence for each determinant.
Their review identifies several key categories of muscle quality determinants. Morphological factors include muscle volume, physiological cross-sectional area (PCSA), fascicle length, and pennation angle — all of which influence the mechanical advantage and force-generating capacity of individual muscles. Neural factors, including motor unit recruitment rate, discharge frequency, synchronization, and neuromuscular efficiency, determine how effectively the nervous system drives muscular contraction. Biochemical and cellular factors — including myosin heavy chain isoform composition (the balance of fast-twitch Type II and slow-twitch Type I fibers), mitochondrial density, oxidative enzyme activity, and intramuscular connective tissue content — modulate both contractile speed and endurance.
Kuschel et al. (2022) also highlight the role of intramuscular fat (IMAT) as a key negative determinant of muscle quality. Elevated IMAT infiltration, which is associated with aging, obesity, insulin resistance, and disuse, impairs force transmission, disrupts cellular signaling, and contributes to chronic low-grade inflammation that further degrades contractile tissue. Ultrasound-derived echo intensity, a non-invasive surrogate for IMAT, emerges from their review as a promising and accessible tool for routine muscle quality assessment in clinical settings.
The review ultimately argues that no single measure captures the full complexity of muscle quality, and that future research should aim to develop composite indices that integrate morphological, neural, and biochemical data into clinically useful scores.
Key Takeaway
Kuschel et al. (2022) establish an important conceptual foundation: muscle quality is not one thing but many, and its determinants operate across structural, neural, and biochemical levels simultaneously. This matters practically because it means that improving muscle quality requires interventions that target multiple systems — resistance training to enhance morphology and neural drive, aerobic conditioning to optimize metabolic capacity, and nutritional strategies to reduce intramuscular fat accumulation. Single-modality approaches are unlikely to be sufficient.
Study 4: Muscle Quality as a Biomarker for Performance in Young Populations
While much of the muscle quality literature focuses on aging, Naimo, Varanoske, Hughes, and Pasiakos (2021), writing in Frontiers in Physiology, make a compelling case that muscle quality is an equally important biomarker for physical performance in younger populations — including military personnel, athletes, and active young adults.
Their narrative review examines evidence from studies using magnetic resonance imaging (MRI), ultrasound, computed tomography (CT), and muscle biopsy data alongside functional performance assessments in young cohorts. They find that even among young adults with equivalent muscle mass, those with higher muscle quality — characterized by greater PCSA, lower intramuscular fat content, higher Type II fiber cross-sectional area, and superior neuromuscular activation — consistently demonstrate better performance on strength, power, endurance, and agility tasks.
Particularly relevant is the authors' discussion of military operational performance. In military populations, where physical demands are extreme and failure of physical performance has serious consequences, body composition metrics that predict performance under load — including weighted marching, obstacle course completion, and fatigue resistance — are of paramount importance. Naimo et al. (2021) present evidence that muscle quality indices derived from ultrasound and BIA-based approaches correlate more strongly with these operationally relevant outcomes than does lean mass alone.
The review also introduces an important developmental perspective: muscle quality is not static across the young adult lifespan. Training status, nutritional adequacy (particularly protein intake and leucine availability), sleep, and hormonal milieu all influence muscle quality trajectories even in individuals aged 18–35. This means that the window for optimizing muscle quality is not confined to old age — investing in muscle quality early in life may pay significant dividends in later decades.
Key Takeaway
Naimo et al. (2021) broaden the relevance of muscle quality beyond geriatrics and establish it as a meaningful biomarker across the full adult age spectrum. For coaches, sports scientists, and military fitness professionals, the takeaway is clear: physical performance assessments and training programs should be designed to assess and optimize muscle quality — not just hypertrophy — from the very beginning of an athlete's or soldier's career. The habits and training adaptations formed in youth lay the structural and biochemical groundwork for muscle quality decades later.
Converging Themes: What the Evidence Collectively Tells Us
1. The Era of Muscle Mass Is Ending
For decades, muscle mass has served as the dominant biomarker of muscular health. It is measurable, quantifiable, and easily standardized through DXA and BIA. But convenience is not the same as clinical relevance.
Emerging data across gerontology, endocrinology, and sports physiology now show that muscle quantity alone fails to predict strength, mobility, metabolic health, or resilience to disease. Two individuals with identical appendicular skeletal muscle mass can display dramatically different functional capacities. The differentiator is not size — it is muscle quality.
2. Muscle Quality: A Functional Paradigm Shift
Muscle quality reframes skeletal muscle as a functional organ system, not merely tissue volume. It integrates:
Strength-to-mass ratio
Neuromuscular efficiency
Intramuscular fat infiltration (myosteatosis)
Mitochondrial density
Fiber-type composition
Muscle architecture (PCSA, pennation angle, fascicle length)
This multidimensional construct explains why strength declines more rapidly than mass with aging — and why metabolic disease accelerates functional impairment even when lean mass appears preserved. In short: mass is anatomy; quality is physiology.
3. Sarcopenia Must Be Redefined
Current sarcopenia frameworks still lean heavily on low muscle mass thresholds. This approach is increasingly inadequate.
Older adults often present with:
Preserved or mildly reduced muscle mass
Significant intramuscular fat infiltration
Reduced specific force
Impaired neuromuscular activation
Mass-based screening can therefore misclassify at-risk individuals as “normal.”
If we are serious about preventing falls, frailty, and disability, diagnostic algorithms must integrate echo intensity, strength-to-mass ratios, and functional performance metrics. The clinical question should shift from “How much muscle is there?” to “How well does it work?”
4. Intramuscular Fat: The Silent Saboteur
Among all determinants of muscle quality, intramuscular adipose tissue (IMAT) stands out as a central pathological driver.
IMAT:
Disrupts force transmission
Promotes chronic low-grade inflammation
Impairs insulin signaling
Reduces contractile efficiency
It explains the paradox of the “normal-weight but weak” individual. It also links metabolic disease, sedentary behavior, and aging under a unified mechanistic framework.
Combating IMAT requires:
Progressive resistance training
Aerobic conditioning
Adequate protein intake
Metabolic control in diabetes and obesity
Muscle quality is, therefore, deeply intertwined with cardiometabolic medicine.
5. Neural Efficiency: The Overlooked Variable
Strength is not simply a product of muscle cross-sectional area. It is a function of:
Motor unit recruitment
Firing frequency
Synchronization
Neuromuscular junction integrity
Age-related decline in neural drive contributes significantly to reduced specific force. Yet most hypertrophy-centred programs neglect neural adaptation.
Explosive resistance training, velocity-based training, and high-intent contractions can restore neuromuscular efficiency — often improving performance without substantial mass gain.
This is why smaller athletes frequently outperform larger but less neurologically efficient counterparts.
6. Muscle Quality Across the Lifespan
Muscle quality is not a geriatric concept. It is relevant from early adulthood onward.
In young populations:
It predicts strength and power output
It correlates with military operational performance
It influences injury resilience
It determines endurance capacity under load
Critically, the trajectory of muscle quality begins early. Poor sleep, inadequate protein intake, sedentary behavior, and metabolic dysfunction in the 20s and 30s may set the stage for accelerated decline decades later. Healthy aging begins with early-life optimization.
7. Implications for Clinical Practice
Healthcare systems must evolve beyond body composition reports that emphasize lean mass percentages without functional context.
A modern muscular health assessment should include:
Handgrip strength normalized to mass
Gait speed or SPPB
Ultrasound echo intensity where available
Resistance training history
Protein adequacy assessment
Preventive medicine must incorporate muscle function as a core vital sign.
8. Implications for Training and Performance Science
The hypertrophy-centric culture of fitness requires recalibration.
Effective muscle quality programs integrate:
Progressive overload for PCSA
Neural adaptation strategies
Aerobic conditioning for mitochondrial density
Periodized intensity
Recovery optimization
Tracking progress through strength-to-bodyweight ratios and performance metrics, rather than scale weight alone, aligns training with functional outcomes.
9. The Metabolic Dimension
Skeletal muscle is the largest site of glucose disposal in the human body. Poor muscle quality contributes to:
Insulin resistance
Reduced glucose uptake
Increased cardiometabolic risk
Thus, improving muscle quality is not merely about strength — it is about metabolic resilience and disease prevention.
10. The Way Forward
We stand at a crossroads. Medicine and fitness can continue to measure what is easy — or begin measuring what matters.
The evidence is converging toward a simple but transformative conclusion:
Quantity without quality is insufficient.
Strength per unit tissue predicts function better than size alone.
Muscle is a metabolic and neurological organ, not just a structural one.
The future of muscular health lies not in building bigger bodies — but in cultivating more efficient, metabolically robust, neurologically integrated muscle systems.
To optimize muscle quality, training must move beyond simple hypertrophy (muscle size) and target the specific structural and neural adaptations identified in recent literature.
Pro-Level Training Modalities for Muscle Quality
Eccentric Overload (Structural Integrity): Emphasizing the lowering phase of a lift increases fascicle length and improves the pennation angle. This enhances the muscle's mechanical advantage and its ability to generate force at longer lengths, which is critical for injury prevention in aging.
Power & Velocity Training (Neural Drive): Lifting lighter loads (30–60% of 1-rep max) with maximal intent and speed specifically recruits Type II (Fast-Twitch) motor units. These are the first fibers lost to age-related decline (sarcopenia).
Isometric Holds (Tendon & Force Transmission): High-tension holds improve tendon stiffness and the efficiency of the "biological pulleys" that transmit force from the muscle to the bone, ensuring no energy is "leaked" during movement.
Multi-Planar Coordination (Neuromuscular Efficiency): Moving through various planes (lateral, rotational) improves motor unit synchronization. This "cleans up the signal" from the brain to the muscle, improving the strength-to-mass ratio without requiring extra bulk.
Metabolic Conditioning (Mitochondrial Quality): Short bursts of high-intensity intervals (HIIT) increase mitochondrial density, reducing intramuscular fat (myosteatosis) and ensuring the muscle has the energy to sustain high-quality contractions.
Frequently Asked Questions (FAQs)
1. What is the difference between muscle mass and muscle quality? Muscle mass refers to the total amount of muscle tissue in the body, typically measured via DXA or BIA as appendicular skeletal muscle mass (ASM). Muscle quality, by contrast, refers to how functionally effective that tissue is — most commonly expressed as the ratio of strength to mass, but also encompassing fiber type composition, intramuscular fat content, neuromuscular efficiency, and metabolic capacity (Kuschel et al., 2022; Rizka et al., 2025).
2. Can someone have high muscle mass but poor muscle quality? Absolutely. This is one of the core insights emerging from this field of research. An older adult who has maintained muscle mass through diet but leads a sedentary lifestyle may have significant intramuscular fat infiltration, poor neuromuscular activation, and low specific force — all hallmarks of poor muscle quality despite adequate mass (Rizka et al., 2025; Kim et al., 2025).
3. How is muscle quality measured in clinical practice? Common methods include the strength-to-mass ratio (e.g., handgrip strength divided by ASM), ultrasound echo intensity as a proxy for intramuscular fat content, the Short Physical Performance Battery (SPPB), and specific force derived from isokinetic dynamometry. MRI and CT can provide more detailed assessments but are less accessible in routine clinical settings (Kuschel et al., 2022; Naimo et al., 2021).
4. Does muscle quality decline with age? Yes. Age-related changes including selective atrophy of fast-twitch (Type II) muscle fibers, increased intramuscular fat infiltration, declining neuromuscular drive, reduced mitochondrial density, and changes in muscle architecture all contribute to a decline in muscle quality that often precedes or parallels the decline in muscle mass (Rizka et al., 2025).
5. Can exercise improve muscle quality? Yes, and this is one of the most clinically important findings in this literature. Resistance training improves neuromuscular efficiency, increases Type II fiber cross-sectional area, and can reduce intramuscular fat. Aerobic exercise enhances mitochondrial density and oxidative capacity. Combined training approaches have the greatest impact on overall muscle quality indices (Kuschel et al., 2022; Naimo et al., 2021).
6. Why is muscle quality particularly important for people with diabetes? People with type 2 diabetes experience accelerated intramuscular fat accumulation, insulin resistance at the muscular level, and peripheral neuropathy — all of which impair muscle quality. Kim et al. (2025) found that high muscle quality can significantly attenuate the negative effects of diabetes on physical performance, making it a particularly important target in the management of this condition.
7. Is muscle quality relevant for young, healthy adults — or just older people? Both. While age-related sarcopenia is a primary driver of interest in muscle quality, Naimo et al. (2021) demonstrate that muscle quality is a significant determinant of physical performance in young adults, including military personnel and athletes. Furthermore, habits and training adaptations formed in youth influence the muscle quality trajectory across the entire lifespan, meaning early investment in muscle quality pays long-term dividends.
✍️ Author’s Note
As a physician trained in internal medicine and deeply engaged in metabolic and exercise science research, I have long observed a critical gap between what we measure and what truly matters in muscular health. For decades, muscle mass has dominated clinical conversations — largely because it is easy to quantify. But clinical experience and emerging evidence increasingly show that function, efficiency, and metabolic integrity tell a far more meaningful story.
This article was written to bridge that gap.
The concept of muscle quality represents a paradigm shift — from viewing muscle as static tissue to understanding it as a dynamic, metabolically active organ system that integrates neural drive, mitochondrial health, fiber composition, and intramuscular fat regulation. In both aging adults and high-performance populations, I have seen firsthand that strength, resilience, and independence are determined less by size and more by functional capacity.
My goal is not simply to summarize research, but to encourage a reframing of how clinicians, fitness professionals, and individuals approach muscular health. If we begin measuring and training for quality over quantity, we can better prevent frailty, mitigate metabolic disease, and enhance lifelong performance.
The future of healthy aging lies not in preserving bulk — but in optimizing biological function.
Medical Disclaimer
The information in this article, including the research findings, is for educational purposes only and does not constitute medical advice, diagnosis, or treatment. Before starting a exercise program, you must consult with a qualified healthcare professional, especially if you have existing health conditions (such as cardiovascular disease, uncontrolled hypertension, or advanced metabolic disease). Exercise carries inherent risks, and you assume full responsibility for your actions. This article does not establish a doctor-patient relationship.
Related Articles
References
Kim, J. A., Shin, C., Jung, I., Park, S. Y., Lee, D. Y., Yu, J. H., Cho, H., Lee, S. K., Kim, K. J., Song, E., Kim, K. J., Kim, N. H., Yoo, H. J., Kim, S. G., Choi, K. M., Kim, N. H., & Seo, J. A. (2025). Impact of muscle quality on muscle strength and physical performance beyond muscle mass or diabetes status. Journal of Cachexia, Sarcopenia and Muscle, 16(2), e13760. https://doi.org/10.1002/jcsm.13760
Kuschel, L. B., Sonnenburg, D., & Engel, T. (2022). Factors of muscle quality and determinants of muscle strength: A systematic literature review. Healthcare, 10(10), 1937. https://doi.org/10.3390/healthcare10101937
Naimo, M. A., Varanoske, A. N., Hughes, J. M., & Pasiakos, S. M. (2021). Skeletal muscle quality: A biomarker for assessing physical performance capabilities in young populations. Frontiers in Physiology, 12, 706699. https://doi.org/10.3389/fphys.2021.706699
Rizka, M., Pohan, R. A., & Andrianie, S. (2025). Beyond quantity: Muscle quality as the cornerstone for sarcopenia diagnosis and healthy aging. Experimental Gerontology, 212, Article 112956. https://doi.org/10.1016/j.exger.2025.112956