Is a Functional Cure for Diabetes Within Reach? The Science of Beta-Cell Restoration
:Can the body regrow insulin-producing cells? Dive into the science of beta-cell restoration for Type 1 and Type 2 diabetes and see how new clinical trials are changing lives.
DIABETES
Dr. T.S. Didwal, M.D.(Internal Medicine)
12/31/202513 min read
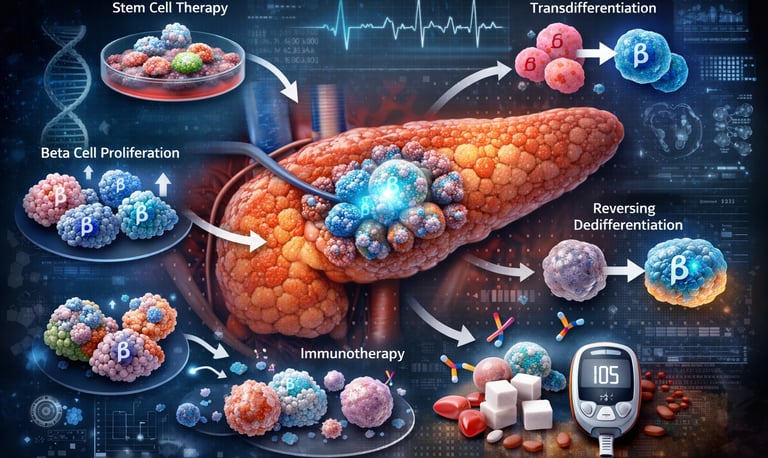
For millions living with diabetes, the daily ritual of finger pricks and insulin calculations feels like a permanent sentence. However, a profound shift is occurring in laboratories worldwide: we are moving from merely managing symptoms to mastering pancreatic beta-cell regeneration. The fundamental challenge in both Type 1 and Type 2 diabetes is the loss or failure of beta cells—the body’s only source of insulin.1 While these cells were once thought to be irreplaceable, modern science suggests the pancreas possesses a hidden "regenerative toolkit" that we are finally learning to unlock.
Recent breakthroughs have identified multiple pathways to restoration, ranging from the use of pluripotent stem cells to "reawakening" dormant cells within the patient's own body (Welters & Lammert, 2022). Perhaps most exciting is the discovery that dysfunctional cells in Type 2 diabetes haven't necessarily died; they have simply "forgotten" their identity, a state known as dedifferentiation that may be entirely reversible (Son & Accili, 2023). By combining chemical biology with advanced immunology, researchers are now entering clinical phases that aim to restore natural glucose control (Karampelias et al., 2025). This isn't just a marginal improvement in care—it is the pursuit of a functional cure.
It is incredibly encouraging to see the shift from theoretical lab work to actual human trials. To give you a clear picture of what the "front lines" look like, here are five clinical approaches currently being tested in humans. These represent the transition from managing symptoms to actually modifying the disease.
Clinical Pearls
1. The "Cellular Shield": Stem Cell-Derived Therapy
This approach uses lab-grown beta cells derived from stem cells. Because the immune system in Type 1 diabetes is programmed to attack these cells, researchers are testing "encapsulation."
The Science: Imagine putting the new cells in a high-tech "tea bag." The pores are small enough to keep immune cells out but large enough to let insulin and glucose flow freely.
Patient Impact: If successful, this could provide a near-infinite supply of insulin-producing cells without the need for lifelong immunosuppressant drugs.
2. The "Molecular Awakening": DYRK1A Inhibitors
Research like the Karampelias et al. (2025) study highlights drugs that can force existing beta cells to multiply—something they usually stop doing in adulthood.
The Science: Scientists identified a "brake" in the cell called DYRK1A. Clinical trials are testing small-molecule drugs (like Harmine) that temporarily "cut the brakes," allowing your own remaining beta cells to divide and grow.
Patient Impact: This is a pill-based or injectable approach that uses your body’s own biology rather than a transplant.
3. "Identity Rescue": Reversing Dedifferentiation
In Type 2 diabetes, cells often stop working because they are "burned out" and lose their identity. This is known as dedifferentiation.
The Science: Clinical trials are investigating whether intensive, early intervention—using a combination of advanced GLP-1 medications and metabolic "rest" periods—can help these cells "remember" how to be beta cells again.
Patient Impact: This could lead to long-term remission for Type 2 patients, moving them away from increasing doses of medication toward a stable, self-regulating pancreas.
4. "The Switch": Alpha-to-Beta Transdifferentiation
The pancreas contains other cells, like alpha cells (which produce glucagon). Interestingly, alpha and beta cells are like "cousins."
The Science: Researchers are testing whether certain compounds can "reprogram" alpha cells to flip a genetic switch and transform into functional beta cells.
Patient Impact: Since the body has plenty of alpha cells, this creates a built-in reservoir of potential insulin producers that the body doesn't see as "foreign."
5. "Hybrid Protection": T-Reg Cell Therapy
For Type 1 diabetes, regeneration isn't enough; you also have to stop the "fire" (the immune attack).
The Science: This clinical approach involves harvesting a patient’s own Regulatory T-cells (T-regs)—the "peacekeeper" cells of the immune system—expanding them in a lab, and infusing them back into the patient.
Patient Impact: By calming the immune attack, this creates a safe environment for regenerated or transplanted beta cells to survive and thrive long-term.
Beta-Cell Regeneration: Breaking New Ground in Diabetes Treatment
Understanding the Beta-Cell Crisis: Why This Matters
Before we explore the solutions, let's understand the problem. Your pancreatic beta cells are the specialized cells responsible for producing insulin—the hormone that regulates blood sugar levels. In Type 1 diabetes, the immune system mistakenly destroys these cells. In Type 2 diabetes, these cells become exhausted and dysfunctional over time, unable to meet your body's insulin demands.
The result? A lifelong dependency on external insulin or medications, constant blood sugar monitoring, and the risk of serious complications like heart disease, kidney failure, and nerve damage.
But what if we could regenerate beta cells or restore their function? That's exactly what researchers are working toward.
The Landscape of Beta-Cell Regeneration Research
Study 1: Novel Approaches to Restore Beta-Cell Mass and Function (Welters & Lammert, 2022)
Welters and Lammert (2022) provide a comprehensive overview of innovative strategies to restore beta-cell mass and improve their function. Published in the Handbook of Experimental Pharmacology, this foundational work explores multiple avenues for diabetes therapy.
Key Approaches Discussed:
Stem Cell Therapy: Using pluripotent stem cells to generate new beta cells in the laboratory, which can then be transplanted into patients
Beta-Cell Proliferation: Encouraging existing beta cells to multiply and regenerate naturally
Transdifferentiation: Converting other pancreatic cell types (like alpha cells or ductal cells) into functional beta cells
Protecting Existing Beta Cells: Implementing strategies to preserve remaining beta-cell function and prevent further loss
Key Takeaways:
Multiple regenerative pathways exist, each with unique advantages
Combination approaches may prove most effective
Both cell replacement and cell restoration strategies show promise
Clinical translation requires overcoming significant technical challenges
This research establishes that beta-cell regeneration isn't a single approach but rather a toolkit of complementary strategies (Welters & Lammert, 2022).
Study 2: Advancing Diabetes Management Through Beta-Cell Restoration (Abdalla, 2024)
Abdalla (2024) takes a more recent look at the field, examining both the tremendous potential and the real-world challenges of pancreatic beta-cell restoration. Published in the World Journal of Gastroenterology, this review balances optimism with practical considerations.
Major Points:
Current Limitations: Existing diabetes treatments manage symptoms but don't address the underlying beta-cell deficit
Regenerative Medicine Promise: New approaches could fundamentally change diabetes from a chronic condition to a potentially curable disease
Clinical Barriers: Issues like immune rejection, maintaining long-term cell viability, and ensuring proper glucose responsiveness remain significant hurdles
Personalized Approaches: Different diabetes patients may require tailored regeneration strategies based on their specific condition
Key Takeaways:
Beta-cell restoration represents a paradigm shift from symptom management to actual disease modification
Success requires addressing both quantity (cell mass) and quality (cell function)
Immunological considerations are critical, especially for Type 1 diabetes patients
Progress is accelerating but clinical applications require careful validation
Abdalla (2024) emphasizes that while the science is advancing rapidly, translating laboratory successes into safe, effective treatments for patients requires rigorous testing and refinement.
Study 3: Reversing Beta-Cell Dedifferentiation in Type 2 Diabetes (Son & Accili, 2023)
This fascinating study by Son and Accili (2023) in Experimental & Molecular Medicine introduces a concept that might surprise you: in Type 2 diabetes, beta cells don't always die—they sometimes just "forget" how to be beta cells.
The Dedifferentiation Phenomenon:
When beta cells become overstressed by constantly high blood sugar levels and insulin resistance, they undergo dedifferentiation—essentially losing their identity and function. They stop producing adequate insulin not because they're destroyed, but because they've transformed into a dysfunctional state.
Reversal Strategies:
Transcription Factor Restoration: Reactivating key genes that maintain beta-cell identity (particularly FOXO1, PDX1, and MAFA)
Metabolic Intervention: Reducing the metabolic stress that triggers dedifferentiation in the first place
Epigenetic Modulation: Targeting the chemical modifications on DNA that control gene expression
Key Takeaways:
Many "lost" beta cells in Type 2 diabetes may actually be recoverable
Dedifferentiation is potentially reversible, unlike cell death
Early intervention may prevent beta cells from losing their identity
This approach could work synergistically with existing diabetes medications
Son and Accili (2023) suggest that reversing beta-cell dedifferentiation might be more achievable than generating entirely new beta cells, offering a more immediate therapeutic pathway.
Study 4: Harnessing Beta-Cell Regeneration Biology for Diabetes Therapy (Bourgeois et al., 2024)
Bourgeois et al.(2024) published an extensive review in Trends in Endocrinology & Metabolism that synthesizes our current understanding of beta-cell regeneration biology and its therapeutic applications.
Regeneration Mechanisms Explored:
Replication of Existing Beta Cells: Stimulating mature beta cells to divide and multiply
Neogenesis: Forming new beta cells from pancreatic progenitor cells
Cellular Reprogramming: Converting non-beta cells into insulin-producing cells
Enhancing Beta-Cell Survival: Protecting cells from apoptosis (programmed cell death)
Molecular Pathways:
The review identifies critical signaling pathways involved in beta-cell regeneration:
Wnt signaling pathway: Promotes cell proliferation and differentiation
Notch pathway: Controls cell fate decisions during development
mTOR pathway: Regulates cell growth and metabolism
STAT3 signaling: Influences cell survival and proliferation
Key Takeaways:
Natural beta-cell regeneration occurs in humans but at extremely low rates
Multiple molecular targets can be manipulated to enhance regeneration
Age, metabolic state, and genetic factors influence regenerative capacity
Combination therapies targeting multiple pathways may yield best results
Bourgeois et al. (2024) emphasize that understanding the fundamental biology of regeneration is essential for developing effective clinical interventions.
Study 5: Mechanistic Insights and Approaches for Beta-Cell Regeneration (Karampelias et al., 2025)
In one of the most recent additions to the field, Karampelias and colleagues (2025) published a comprehensive analysis in Nature Chemical Biology that dives deep into the molecular mechanisms driving beta-cell regeneration.
Chemical and Biological Approaches:
Small Molecule Therapies: Identifying chemical compounds that can stimulate beta-cell proliferation without compromising function
Growth Factor Signaling: Leveraging natural growth factors like EGF (epidermal growth factor) and HGF (hepatocyte growth factor)
Metabolic Reprogramming: Altering cellular metabolism to favor regeneration
MicroRNA Regulation: Using small RNA molecules to control gene expression patterns
Drug Development Insights:
The study highlights several promising drug candidates in various stages of development:
Harmine and related compounds that inhibit DYRK1A (a protein that normally suppresses beta-cell replication)
GLP-1 receptor agonists that not only improve insulin secretion but may also promote beta-cell survival
GABA (gamma-aminobutyric acid) which shows potential for protecting and regenerating beta cells
Key Takeaways:
Chemical biology approaches offer practical therapeutic pathways
Several compounds have shown efficacy in animal models
Understanding mechanism is crucial for optimizing drug effects
Clinical trials are beginning to test some of these approaches in humans
Karampelias et al. (2025) bridge the gap between basic science and clinical application, offering concrete examples of how laboratory discoveries are moving toward patient treatments.
Study 6: Harnessing Beta-Cell Replication for Regenerative Therapies (Vasavada & Dhawan, 2025)
The most recent study in our review, by Vasavada and Dhawan (2025) in Frontiers in Endocrinology, focuses specifically on beta-cell replication—getting existing beta cells to divide and multiply.
Why Replication Matters:
Unlike generating entirely new cells from stem cells, stimulating existing beta cells to replicate offers several advantages:
The cells are already properly differentiated and functional
They're in their natural environment within the pancreatic islets
They maintain appropriate connections with blood vessels and other cell types
Replication Strategies:
Cell Cycle Regulators: Targeting proteins that control whether cells divide (cyclins, CDKs)
Menin Inhibitors: Blocking a protein that normally prevents beta-cell replication
Age-Dependent Approaches: Recognizing that beta-cell replication capacity changes with age
Pregnancy-Related Mechanisms: Learning from the natural beta-cell expansion that occurs during pregnancy
Clinical Applications:
Vasavada and Dhawan (2025) discuss how understanding replication mechanisms can inform treatment timing and patient selection:
Younger patients may respond better to replication-based therapies
Early intervention in prediabetes might prevent full diabetes development
Combining replication stimulation with immune modulation could benefit Type 1 diabetes patients
Key Takeaways:
Beta-cell replication naturally occurs but declines with age and disease progression
Multiple molecular brakes on replication can be therapeutically targeted
Timing interventions appropriately is critical for success
Pregnancy studies provide valuable insights into natural beta-cell expansion
This research emphasizes that we already have templates for successful beta-cell expansion in nature—we just need to learn how to recreate them therapeutically (Vasavada & Dhawan, 2025).
Synthesizing the Research: What Does This Mean for Diabetes Treatment?
When we look at these six studies together, several powerful themes emerge:
1. Multiple Pathways to Beta-Cell Restoration
There isn't just one way to restore beta cells. Whether through regeneration, replication, reprogramming, or dedifferentiation reversal, researchers have identified numerous potential approaches. This diversity increases the likelihood that effective treatments will emerge.
2. Personalized Medicine Approaches
Different patients may benefit from different strategies. A newly diagnosed Type 1 diabetes patient with recent beta-cell loss might benefit most from stem cell replacement, while a Type 2 diabetes patient might respond better to dedifferentiation reversal or proliferation stimulation.
3. Combination Therapies Show Promise
Rather than relying on a single approach, the future likely involves combination strategies that simultaneously protect existing cells, stimulate regeneration, and modulate immune responses.
4. Moving From Bench to Bedside
The field has progressed from purely theoretical to increasingly practical. Several approaches are now in clinical trials, meaning we're moving closer to actual treatments that patients can access.
Challenges That Remain
Despite this exciting progress, significant obstacles remain:
Immune System Concerns: For Type 1 diabetes patients, any regenerated or replaced beta cells face the same immune attack that destroyed the original cells. This requires concurrent immunomodulation or encapsulation technologies to protect new cells.
Long-Term Functionality: Lab-grown or regenerated beta cells must function properly for years or decades, responding appropriately to blood sugar changes without losing effectiveness.
Safety Considerations: Stimulating cell proliferation carries inherent risks, including potential tumor formation. Any therapy must be carefully designed to promote beta-cell growth specifically without affecting other cell types.
Scalability and Cost: Many promising approaches are complex and expensive. Translating them into treatments accessible to millions of diabetes patients worldwide requires addressing manufacturing, cost, and delivery challenges.
Regulatory Pathways: Novel cellular therapies face rigorous regulatory scrutiny (appropriately so), which can extend the timeline from discovery to approval.
What Can You Do Right Now?
While these breakthrough treatments are being developed, here's how you can take action today:
Stay Informed
Follow reputable diabetes research organizations like JDRF, ADA (American Diabetes Association), and Diabetes UK
Discuss emerging therapies with your healthcare provider
Consider participating in clinical trial registries if you're interested in experimental treatments
Optimize Current Management
Work with your healthcare team to achieve the best possible blood sugar control
This helps preserve remaining beta-cell function
Good management today may leave you in better position to benefit from regenerative therapies tomorrow
Support Research
Consider donating to diabetes research organizations
Participate in research studies if eligible
Advocate for increased research funding
Lifestyle Factors
Maintain a healthy weight and exercise regularly—these lifestyle interventions can reduce stress on remaining beta cells
Certain dietary patterns (like Mediterranean diet) show benefits for metabolic health
Stress management may also support overall metabolic function
Frequently Asked Questions
Q: When will beta-cell regeneration treatments be available?
A: Some approaches are already in early clinical trials. However, the path from promising research to widely available treatment typically takes 5-15 years. The most optimistic estimates suggest certain therapies could reach patients within the next 5-10 years, though this timeline depends on trial results and regulatory approval.
Q: Will these treatments cure diabetes?
A: The goal is functional cure—restoring normal insulin production without need for external insulin. However, "cure" may look different for Type 1 versus Type 2 diabetes. Type 1 patients may need ongoing immune suppression, while Type 2 patients might achieve lasting remission if underlying metabolic issues are addressed.
Q: How much will these treatments cost?
A: Cost remains uncertain and will vary by approach. Stem cell therapies and sophisticated cellular engineering may initially be expensive, but costs typically decrease as technologies mature. Many researchers are working specifically on scalable, cost-effective approaches to ensure broad accessibility.
Q: Are these treatments safe?
A: Safety is rigorously evaluated throughout the drug development process. Early-stage trials focus heavily on safety before effectiveness. Any approved therapy will have undergone extensive testing to ensure benefits outweigh risks.
Q: Can I still benefit if I've had diabetes for many years?
A: Potentially yes. Even patients with long-standing diabetes may have some remaining beta cells or regenerative capacity. However, earlier intervention generally offers better outcomes, emphasizing the importance of early diagnosis and good management.
Q: Will my insurance cover these treatments?
A: Coverage depends on regulatory approval, demonstrated effectiveness, and specific insurance policies. As treatments become standard of care, insurance coverage typically follows, though this process takes time.
Key Takeaways
✓ Beta-cell regeneration represents the most promising frontier in diabetes research, offering hope for functional cure rather than just management
✓ Multiple complementary approaches—including stem cell therapy, cellular reprogramming, replication stimulation, and dedifferentiation reversal—are advancing simultaneously
✓ Recent research (Welters & Lammert, 2022; Abdalla, 2024; Son & Accili, 2023; Bourgeois et al., 2024; Karampelias et al., 2025; Vasavada & Dhawan, 2025) demonstrates both the biological feasibility and increasing clinical practicality of beta-cell restoration
✓ Both Type 1 and Type 2 diabetes patients may benefit from regenerative approaches, though strategies may differ
✓ Several therapeutic candidates are progressing toward clinical trials, suggesting treatments could become available within the next decade
✓ Current diabetes management remains crucial and may preserve regenerative capacity for future therapies
✓ Personalized medicine approaches will likely match specific regeneration strategies to individual patient characteristics
The Road Ahead: Hope Grounded in Science
The convergence of insights from Welters and Lammert (2022), Abdalla (2024), Son and Accili (2023), Bourgeois et al. (2024), Karampelias et al. (2025), and Vasavada and Dhawan (2025) paints a picture of a field rapidly maturing from concept to reality.
We're witnessing a transformation in how we think about diabetes—from an irreversible condition requiring lifelong management to a potentially reversible disease where beta-cell restoration could offer lasting solutions.
The science is advancing faster than ever before. New discoveries about cellular plasticity, regenerative mechanisms, and molecular pathways are opening therapeutic doors we couldn't have imagined a decade ago. While challenges remain, the trajectory is clear: beta-cell regeneration therapy is not just a distant dream but an emerging reality.
For the millions living with diabetes today, this research represents genuine hope—hope grounded in rigorous science, supported by compelling data, and driven by dedicated researchers worldwide who refuse to accept that diabetes must be a lifelong sentence.
Take Action Today
Stay Connected: Subscribe to updates from leading diabetes research organizations to stay informed about the latest breakthroughs.
Talk to Your Doctor: Discuss how emerging regenerative therapies might factor into your long-term treatment plan.
Consider Clinical Trials: Visit ClinicalTrials.gov to explore whether you might be eligible for trials testing novel beta-cell regeneration approaches.
Support the Cause: Whether through donations, advocacy, or participation in research, every contribution accelerates progress toward effective treatments.
Maintain Hope and Commitment: Continue managing your diabetes effectively today while looking forward to the transformative treatments on the horizon.
The future of diabetes treatment isn't just about better management—it's about restoration, regeneration, and ultimately, freedom from the disease itself
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Individual circumstances vary, and treatment decisions should always be made in consultation with qualified healthcare professionals.
Related Articles
The Metabolic Triad: Why Diabetes, Obesity & CVD Are One Epidemic | DR T S DIDWAL
What’s New in the 2025 Blood Pressure Guidelines? A Complete Scientific Breakdown | DR T S DIDWAL
Manage Diabetes Naturally: How Beta-Glucans Control Blood Sugar | DR T S DIDWAL
Exercise as Metabolic Medicine: Latest Research on Glucose and Heart Health| DR T S DIDWAL
References
Abdalla, M. M. I. (2024). Advancing diabetes management: Exploring pancreatic beta-cell restoration's potential and challenges. World Journal of Gastroenterology, 30(40), 4339–4353. https://doi.org/10.3748/wjg.v30.i40.4339
Bourgeois, S., Coenen, S., Degroote, L., Willems, L., Van Mulders, A., Pierreux, J., Heremans, Y., De Leu, N., & Staels, W. (2024). Harnessing beta cell regeneration biology for diabetes therapy. Trends in Endocrinology & Metabolism, 35(11), 951–966. https://doi.org/10.1016/j.tem.2024.03.006
Karampelias, C., Liu, K. C., Tengholm, A., Rutter, G. A., Spégel, P., & Artner, I. (2025). Mechanistic insights and approaches for beta cell regeneration. Nature Chemical Biology, 21, 807–818. https://doi.org/10.1038/s41589-024-01822-y
Son, J., & Accili, D. (2023). Reversing pancreatic β-cell dedifferentiation in the treatment of type 2 diabetes. Experimental & Molecular Medicine, 55, 1652–1658. https://doi.org/10.1038/s12276-023-01043-8
Vasavada, R. C., & Dhawan, S. (2025). Harnessing beta-cell replication: Advancing molecular insights to regenerative therapies in diabetes. Frontiers in Endocrinology, 16, Article 1612576. https://doi.org/10.3389/fendo.2025.1612576
Welters, A., & Lammert, E. (2022). Novel approaches to restore pancreatic beta-cell mass and function. Handbook of Experimental Pharmacology, 274, 439–465. https://doi.org/10.1007/164_2021_474
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with qualified healthcare professionals regarding your diabetes management and treatment options.